Demethylation of 2-methoxy-9H-thioxanthen-9-one
SyntheticPage 868
DOI:
Submitted: November 13, 2018, published: November 14, 2018
Authors
Mariyah Sajjad
Mark Wainwright (M.Wainwright@ljmu.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
Robin W.R. van den Kieboom
A contribution from
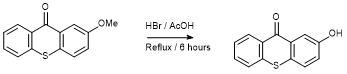
Chemicals
2-Methoxy-9H-thioxanthen-9-one (prepared in house, see Synthetic Page 847)
Hydrobromic acid (Sigma Aldrich)
Glacial Acetic Acid (Fisher Scientific)
Procedure
To a quick fit round bottomed flask charged 2-methoxy-9H-thioxanthen-9-one (2.42 g, 10 mmol), concentrated hydrobromic acid (20 mL) and glacial acetic acid (20 mL) and the reaction mixture was heated to reflux for 6h with constant stirring.* Upon cooling the reaction mixture was poured over ice (200 g) and distilled water (300 mL) was added slowly with vigorous stirring. The yellow precipitate produced was isolated and dried at the vacuum pump to yield 2-hydroxy-9H-thioxanthen-9-one (2.02g, 89%) as a yellow solid.
Author Comments
* A quick fit round bottomed flask and reflux condenser was used, which fitted snugly into a metal heating block. A thermocouple was used to control the temperature and reaction was open to the air (no inert gasses were required). Stirring was accomplished using a stirring bar which was controlled from the hot plate. The whole reaction was accomplished in the open air without the need of dry/inert conditions.
Data
1H NMR (400 MHz, DMSO-d6) δ 8.43 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 2.7 Hz, 1H), 7.80 – 7.68 (m, 2H), 7.68 – 7.63 (m, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.26 (dd, J = 8.7, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 179.04, 156.90, 137.43, 133.07, 130.05, 129.51, 128.46, 128.19, 126.94, 126.82, 126.60, 123.17, 113.67. LCMS m/z: 229 [M+]
Lead Reference
Casimero, C., McConville, A., John-Joe, F., Lawrence, C.L., Taylor, C.M., Smith, R.B., Davis J. Sensor systems for bacterial reactors: A new flavin-phenol composite film for the in situ voltammetric measurement of pH (2018) Analytica Chimica Acta, (2018), 1027, pp. 1-8
Supplementary Information
Keywords
alcohols, aromatics/arenes, demethylation, ethers, ketones, thiol