Ring closure via thermal decarboxylation of 2-(4-methoxyphenyl)thio)benzoic acid to 2-methoxy-9H-thioxanthen-9-one
SyntheticPage 847
DOI:
Submitted: August 23, 2018, published: September 5, 2018
Authors
Joshua Gettins
Mariyah Sajjad
Mark Wainwright (M.Wainwright@ljmu.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
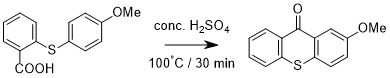
Chemicals
2-((4-methoxyphenyl)thio)benzoic acid (prepared in house, see Synthetic Page 846)
Sulfuric acid (Fisher Scientific)
Isopropyl alcohol (Fisher Scientific)
Procedure
Into a conical flask was charged 2-((4-methoxyphenyl)thio)benzoic acid (2.60 g, 10 mmol) followed by concentrated sulphuric acid (40 mL) in one full portion*. The mixture was heated to 100°C** with constant stirring for 30 minutes. The hot solution was carefully poured over ice (200 g); with vigorous stirring a yellow solid precipitated from the reaction which was isolated by vacuum filtration at the pump and washed further with water (100 mL). The crude solid was recrystallized in the minimum amount of hot isopropyl alcohol to yield 2-methoxy-9H-thioxanthen-9-one (0.61 g, 25%) as bright yellow needles.
Author Comments
*Upon addition of sulphuric acid an exothermic reaction occurs with effervescence. This subsides with heat and stirring to produce a clear brown liquid.
**Temperature was established and maintained using a thermocouple which was placed into a metal heating block. Stirring was accomplished using a standard stirring bar.
Data
1H NMR (400 MHz, Chloroform-d) δ 8.65 (d, J = 8.6 Hz, 1H), 8.10 (d, J = 2.9 Hz, 1H), 7.66 – 7.57 (m, 2H), 7.54 – 7.46 (m, 2H), 7.28 (dd, J = 8.9, 3.1 Hz, 1H), 3.96 (s, 3H).13C NMR (101 MHz, CDCl3) δ 179.70, 158.39, 137.53, 132.03, 130.23, 129.88, 129.19, 128.58, 127.31, 126.10, 126.00, 122.78, 110.33, 55.71. LCMS – 242 [M+]
Lead Reference
Supplementary Information
Keywords
aromatics/arenes, carboxylic acids, dehydration, ketones, thermal