Coupling of 2-bromobenzoic acid with 4-methoxythiophenol under Ullmann conditions
SyntheticPage 846
DOI:
Submitted: August 23, 2018, published: October 15, 2018
Authors
Joshua Gettins
Mariyah Sajjad
Mark Wainwright (M.Wainwright@ljmu.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
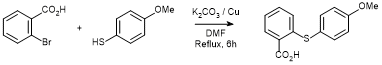
Chemicals
2-Bromobenzoic Acid (Sigma Aldrich)
4-Methoxythiophenol (Sigma Aldrich)
Anhydrous potassium carbonate (Sigma Aldrich)
100 mesh copper bronze (Alfa Aesar)
Procedure
A mixture of 2-bromobenzoic acid (10.63 g, 52.9 mmol), 4-methoxythiophenol (6.57 g, 52.9 mmol), K2CO3 (14.60 g, 105.8 mmol) and powdered Cu bronze (1.71 g, 26.44 mmol) in DMF (100 mL) was stirred at reflux for 6 hours. Upon cooling, the reaction mixture was poured into ice (500 g) and acidified with concentrated HCl to pH=4 giving a white solid. The solid was isolated and dried at the vacuum pump** to yield 2-((4-methoxyphenyl)thio)benzoic acid (6.10 g, 44%) as a white solid.
Author Comments
*** Round bottom flask fitted with a reflux condenser was used which fitted snuggly into a metal heating block. A thermocouple was used to control the temperature and reaction was open to the air (no inert gasses were required). Stirring was accomplished using a stirring bar which was controlled from the hot plate. The whole reaction was accomplished in the open air without the need of dry/inert conditions.
** pH determined using universal indicator paper.
***No purification required, but the authors suggest holding the compound at the vacuum pump for at least an hour until dry.
Data
1H NMR (400 MHz, DMSO-d6) δ 13.27 (s, 1H), 7.93 (dd, J = 7.7, 1.5 Hz, 1H), 7.47 (d, J = 8.5 Hz, 2H), 7.36 – 7.29 (m, 1H), 7.15 (td, J = 7.5, 1.2 Hz, 1H), 7.06 (d, J = 8.6 Hz, 2H), 6.65 (dd, J = 8.3, 1.2 Hz, 1H), 3.81 (s, 3H). 13C NMR (101 MHz, DMSO) δ 167.80, 160.80, 143.95, 137.89, 132.81, 131.42, 127.13, 126.37, 124.57, 122.41, 116.15, 55.77. MS m/z: 261.02 [M+].
Lead Reference
Wang, C., Jiang, H., Jin, J., Xie, Y., Chen, Z., Zhang, H., Lian, F., Liu, Y.-C., Zhang, C., Ding, H., Chen, S., Zhang, N., Zhang, Y., Jiang, H., Chen, K., Ye, F., Yao, Z., Luo, C.
Development of Potent Type i Protein Arginine Methyltransferase (PRMT) Inhibitors of Leukemia Cell Proliferation
Journal of Medicinal Chemistry, (2017): 60 (21), pp. 8888-8905.
Supplementary Information
Keywords
aromatics/arenes, carboxylic acids, nucleophilic, thiol, Ullmann