Reduction of thioxanthen-9-one to produce thioxanthen-9-ol
SyntheticPage 848
DOI:
Submitted: August 23, 2018, published: September 10, 2018
Authors
Joshua Gettins
Mariyah Sajjad
Mark Wainwright (M.Wainwright@ljmu.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
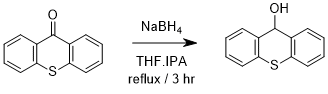
Chemicals
Thioxanthen-9-one (Prepared in house, see Synthetic Page 847)
Sodium borohydride (Sigma Aldrich)
Isopropyl alcohol (IPA) (Fisher Scientific)
Tetrahydrofuran (THF) (Fisher Scientific)
Procedure
A mixture of thioxanthen-9-one (1 g, 4.7 mmol), isopropyl alcohol* (40 mL) and THF (20 mL) was heated to reflux with constant stirring**. Upon a clear solution being obtained***, NaBH4 (1.4 g, 18.5 mmol) was added in small portions carefully, and the reaction was left to stir at reflux for 3 hours. Upon cooling, the solution poured over ice (250 g) and the reaction mixture was stirred at speed until a white solid was produced****. The solid was isolated and dried at the pump under vacuum filtration***** to yield thioxanthen-9-ol (0.77 g, 76%) as a white solid.
Author Comments
* IPA used instead of methanol due to solubility issues with the starting thioxanthen-9-one.
** Round bottom flask fitted with a reflux condenser was used which fitted snuggly into a metal heating block. A thermocouple was used to control the temperature and reaction was open to the air (no inert gasses were required). Stirring accomplished using a stirring bar which was controlled from the hot plate.
*** A clear solution obtained after 10 minutes at reflux, however should this not happen add 5 mL portions of THF (slowly) to the reaction mixture (through the top of the condenser) until a clear solution is obtained.
**** Should only a small amount of white solid be produced upon stirring, the addition of distilled water (approx.. 200mL) in one potion will help precipitation.
*****No purification required, but the authors suggest holding the compound at the vacuum pump until dry. The authors suggest doing this for one hour.
Data
1H NMR (400 MHz, DMSO-d6) δ 7.70 (d, J = 7.7 Hz, 2H), 7.51 (d, J = 7.6 Hz, 2H), 7.41 – 7.34 (m, 2H), 7.31 – 7.24 (m, 2H), 6.45 (d, J = 5.7 Hz, 1H), 5.19 (d, J = 5.6 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 139.43, 131.27, 127.28, 127.05, 126.91, 125.73, 69.75. LCMS = 214 [M+]
Lead Reference
Fujiwara, T., Ohira, K., Urushibara, K., Ito, A., Yoshida, M., Kanai, M., Tanatani, A., Kagechika, H., Hirano, T. Steric structure–activity relationship of cyproheptadine derivatives as inhibitors of histone methyltransferase Set7/9. (2016) Bioorganic and Medicinal Chemistry, 24 (18), pp. 4318-4323.
Supplementary Information
Keywords
alcohols, aromatics/arenes, ketones, reduction