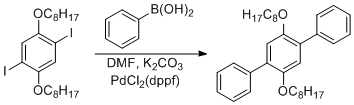
Suzuki coupling of 1,4-dioctyloxy-2,5-diiodobenzene and phenylboronic acid
SyntheticPage 767
DOI:
Submitted: October 3, 2014, published: October 6, 2014
Authors
Garrett Cairo (cairog@berea.edu)
Nicholas Marshall (marshalln@berea.edu)
A contribution from
Chemicals
1,4-dioctyloxy-2,5-diiodobenzene (see References)
PdCl2dppf, Sigma-Aldrich
potassium carbonate, Sigma-Aldrich, 99%
phenylboronic acid, Sigma-Aldrich
Ethyl acetate
0.1M hydrochloric acid
0.1M sodium hydroxide
saturated sodium chloride solution
anhydrous sodium sulfate methanol
dichloromethane
hexane
Procedure
1,4-dioctyloxy-2,5-diiodobenzene (12.90 g, 22 mmol), phenylboronic acid (6.29 g, 49 mmol, 2.2 eq.) potassium carbonate (7.297 g, 52.8 mmol, 2.4 eq), and PdCl2dppf (0.719 g, 0.88 mmol, 0.04 eq) were combined with a magnetic stirbar in a 500-mL 3-neck round-bottom flask with two of the necks stopped with septa and one fitted with a reflux condenser. Dimethylformamide (200 mL) was added, and the suspension was purged by bubbling with nitrogen gas from a long stainless steel needle. The purging was stopped and the reaction placed under normal nitrogen flow, and the reaction was heated at 80°C for 5 h using a mineral oil bath with a thermometer.
The reaction mixture was poured into a 1 L separatory funnel along with ethyl acetate (100 mL) and water (100mL) and swirled thoroughly. The aqueous layer was removed and the remaining organic layer washed twice with water, once with 0.1M hydrochloric acid, once with 0.1M sodium hydroxide, and once with brine. The resulting organic layer was dried over sodium sulfate and rotary evaporated to dryness, yielding a black solid.
The resulting solid was dissolved in a minimal amount of dichloromethane and poured onto a glass fritted funnel under water-aspirator suction that has been loaded with a 1-2 inch plug of flash-chromatographic grade silica gel. (See Author’s Comment) The crude product was eluted from the silica by pouring several portions of 20% dichloromethane in hexanes over the silica under vacuum, using an approximate total of 150-200 mL eluent. The solution in the filtering flask was rotary evaporated to dryness, giving a beige solid. The solid was dissolved in minimal dichloromethane and methanol slowly added until precipitate appeared, followed by cooling in an ice bath, filtration, and washing with cold methanol to give white crystalline product (4.80g, 10.23 mmol, 47% yield).
Author Comments
- We prepared this molecule and several in the same category in the process of synthesizing monomers for conjugated polymer synthesis. The Suzuki coupling, in our hands, is much cleaner for assembling terphenyl derivatives than Kumada coupling. We prepared the 1,4-diiodo starting material using the procedure in the Lead Reference. We have also recently published a Synthetic Page procedure for a simpler and more environmentally friendly preparation of the same material.
- Nitrogen was bubbled through the reaction by sticking a disposable 22-gauge needle into one of the septa as a vent, then sticking the long needle into the base of the flask through the other septum. We then adjusted the nitrogen flow on the manifold to a slow bubbling, opened the valve to the reaction, and capped the exit bubbler with a latex pipet bulb. (This is preferable to shutting off the exit valve entirely, to avoid excess pressure buildup if the needle should become clogged; the pipet bulb is a nice simple pressure-relief valve)
- TLC was checked at several points in the reaction. Product appeared as a purple fluorescent spot on TLC under 254 nm UV light, Rf = 0.6 in 5% ethyl acetate/ hexanes on silica. In solution, the product fluoresces a brilliant blue under 365 nm UV light.
- The “plug column” with the fritted funnel is a crude dry-column vacuum chromatography technique that is better at removing moderately polar impurities than a Celite plug at the cost of some product which remains absorbed. With a colorless product such as this one, we usually add eluent until it appears that colored impurities are about to break through the plug. We also demonstrate this technique in SyntheticPage 759.
- Multiple crops of crystals can be harvested after the first crystallization from DCM/methanol, with diminishing yields each time.
- We used this procedure to prepare the methoxy and ethoxy relatives of this product as well, with no major modifications to the procedure. We have also performed it on a smaller 2.4 mmol scale (of the diiodo coupling partner) with a similar (49%) overall yield.
- We also carried out the reaction with similar results using 4 mol% Pd(OAc)2 and 4 equivalences of triphenyl phosphine relative to the Pd(OAc)2.
Data
1H NMR (CDCl3, δ vs. TMS, 300 MHz): 7.61 (dt, 4H) 7.42 (tt, 4H) 7.33 (tt, 2H) 6.99 (s,2H) 3.91 (t, 4H) 1.68 (p, 4H) 1.25 (m, 20H) 0.88 (t, 6H)
13C INEPT NMR (CDCl3, δ vs. TMS, 75 MHz): 129.5 (CH); 127.8 (CH); 126.8 (CH); 116.3 (CH); 69.6 (CH2O); 31.7 (CH2); 29.1 (CH2); 26.0 (CH2); 22.6 (CH2); 14.1 (CH3).
Melting point 57.0-58.1°C (uncorrected)Lead Reference
N. Marshall, S.K. Sontag, and J. Locklin. Macromolecules, 2010, 43, 2137-2144 http://dx.doi.org/10.1021/ma902710j
Other References
N. Ebai and N. Marshall, ChemSpider SyntheticPages, 2014, http://cssp.chemspider.com/762
Supplementary Information
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes, carbocyclic compounds, Suzuki, transition metal catalysed