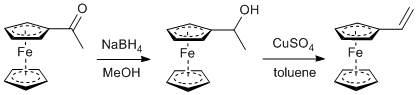
Reduction and dehydration of acetylferrocene
SyntheticPage 759
DOI:
Submitted: August 5, 2014, published: August 14, 2014
Authors
Nicholas Marshall (nicholas_marshall@berea.edu)
Tatiana Mikhailova (tanya-mihailova@mail.ru)
Chemicals
Acetylferrocene, 95% (Fisher Scientific)
Sodium borohydride, 98% (J.T. Baker)
Methanol (Fisher Scientific)
Copper sulfate, 98% (Sigma Aldrich)
Toluene (Fisher Scientific)
Hexanes (Fisher Scientific)
Procedure
Author Comments
- This procedure is based on that of Wang et al. (see Lead Reference) which is commonly cited for the synthesis of vinylferrocene, but actually describes the synthesis of a different vinyl iron complex. To the best of our knowledge, ours is the only procedure which describes the synthesis in detail.
- Acetylferrocene is readily made by the Friedel-Crafts acylation of ferrocene using either acetic anhydride or acetyl chloride; efficient and green procedures for this reaction are freely available online as undergraduate-lab level experiments.
- The reduction was performed using both ethanol and methanol as a solvent, in our experience methanol results in an easier workup of the alcohol but either works acceptably. TLC was used to confirm complete conversion of acetylferrocene to the alcohol; co-spotting was necessary, as the two compounds had similar Rf values in all solvent systems we checked.
- Copper sulfate hexahydrate was powdered in a mortar and pestle and heated until a uniform white color in a 250°C lab drying oven, then stored in a sealed glass jar. A heat gun can also be used to prepare small portions of this drying agent by heating the hydrated compound in a test tube.
- The mass of copper sulfate and reflux time in toluene was not optimized. The reaction could potentially be improved by changing these parameters. Use of a Dean-Stark trap would be ideal.
- Toluene can be readily removed by forced evaporation using a stream of house compressed air. In this procedure, the flask of product dissolved in toluene is clamped in a water bath warmed on a hot plate, and a gentle stream of air from a rubber tube passed into the flask. This procedure is often more convenient for us than rotary evaporation due to the length of time required to remove toluene on the rotovap, but removal of the solvent by rotovap worked in our experience as well.
- We use a crude form of dry-column vacuum chromatography (a "plug column") to avoid the need to run a column on the product. Using hexane as the mobile phase, starting material and a more polar impurity visible on TLC are entirely retained on the small plug of silica gel and do not contaminate the product. Ordinary flash-chromatography grade silica gel is used for this process, which is very convenient in our hands.
- NMR displays significant broadening of all peaks due to Fe(III) present in trace amounts.
Data
1HNMR (300 MHz, DMSO-d6): 6.39 (m, 1H, ppm vs. TMS), 5.31 (doublet of multiplets, 1H), 4.98 (m, 1H), 4.38 (dd, 2H), 4.18 (dd, 2H), 4.04 (m, 5H). 13C NMR (75 MHz, DMSO-d6, ppm vs. TMS): 135, 112, 83, 69, 67
Lead Reference
Wang, Y. Lin, T. Shyu, R. Hwu, J. Wang, Y. Cheng, M. J. Organomet. Chem. 1989, 371(1), 57-69. DOI: 10.1016/0022-328X(89)85207-6
Keywords
alcohols, alkenes, aromatics/arenes, cyclopentadienyl, elimination, ferrocene, hydrogenation, iron(II), ketones, organometallics, reduction
Comments
Other vinyl-Fc prep
Shi et al. published an alternative procedure for the preparation of this molecule at the same time (Chem. Sci. Eng., 2014, 8(2), 171-178.) Their procedure is a fine one and includes the common Friedel-Crafts acylation of ferrocene with acetic anhydride/phosphoric acid, which may be convenient for users of our prep as well. We welcome comments from anyone who has used both procedures.
By Nicholas Marshall on August 19, 2014