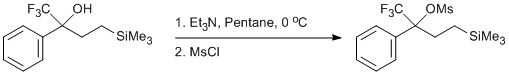
Mesylation of a Trifluoromethyl Carbinol
SyntheticPage 757
DOI:
Submitted: July 11, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
4-(dimethyl(phenyl)silyl)-1,1,1-trifluoro-2-phenylbutan-2-ol (Prepared from 3-(dimethyl(phenyl)silyl)-1-phenylpropan-1-one according the procedure outlined in ChemSpider SyntheticPage 550)
Triethylamine (≥99%, Sigma Aldrich)
Pentane (99+%,n-Pentane, 95% min, Alfa Aesar)
Methanesulfonyl Chloride (≥99.7%, Sigma Aldrich)
Procedure
The following is a modification of the procedure outlined by Crossland et al. and Gassman et al. To a flame dried 50 mL round bottom flask containing a stir bar was added pentane (9 mL), the CF3 alcohol (0.50 g, 1.8 mmol, 1 equiv), and triethylamine (0.273 g, 2.7 mmol, 1.5 equiv) under nitrogen. The reaction flask was placed in an ice bath and allowed to cool to 0 oC for 10 minutes. After this time, MsCl (0.227 g, 1.98 mmol, 1.1 equiv) was added dropwise to the flask. The reaction was allowed to stir for 15 minutes at 0 oC and then warmed to room temperature for 30 minutes. The progress of the reaction was monitored by 1H NMR and deemed incomplete at this time. The reaction was then cooled to 0 oC again and another portion of triethylamine (0.137 g, 1.35 mmol, 0.75 equiv) was added followed by MsCl (0.113 g, 0.99 mmol, 0.55 equiv) dropwise. The reaction was allowed to stir for 15 minutes at 0 oC and then warmed to room temperature for 30 minutes. This process was repeated again, and the reaction deemed complete at this time by 1H NMR.1 The reaction mixture was diluted with pentane (10 mL) and quenched with deionized water (5 mL). The solution was then transferred to a separatory funnel and the aqueous layer extracted with pentane (3 x 20 mL). The combined organic layers were then washed with water (10 mL), saturated sodium bicarbonate (10 mL), and brine (10 mL). The organic layer was dried with Na2SO4 and the solvent removed via rotary evaporation in a 28 oC water bath and then placed under a high vacuum to remove residual solvent to give the desired product (0.641 g, 97%) as a light yellow oil.
Author Comments
.
Data
1H NMR (400 MHz, CDCl3) δ ppm 0.02 (s, 9 H) 0.38 (td, J = 14.00, 3.99 Hz, 1 H) 0.72 (td, J = 14.00, 3.94 Hz, 1 H) 2.31 (td, J = 14.50, 3.70 Hz, 1 H) 2.93 (td, J = 14.00, 3.89 Hz, 1 H) 3.18 (s, 3 H) 7.41 - 7.47 (m, 3 H) 7.48 - 7.53 (m, 2 H) 13C NMR (100 MHz, CDCl3) d ppm -1.91 (CH3) 9.62 (CH2) 27.26 (CH2) 40.85 (CH3) 93.66 (q, JC-C-F = 28.30 Hz, C) 124.03 (q, JC-F = 285.70 Hz, CF3) 126.97 (CH) 128.68 (CH) 129.56 (CH) 133.81 (C) 19F NMR (377 MHz, CDCl3) d ppm –77.93.
Lead Reference
Other References
Crossland, R. K.; Servis, K. L. J. Org. Chem., 1970, 35, 3195
Nelson, D. W.; O’Reilly, N. J. Speier, J.; Gassman, P. G. J. Org. Chem. 1994, 59, 8157
Keywords
alcohols, aromatics/arenes, Leaving Groups, Mesylate, Sulfonate Esters, Trifluoromethyl