Trifluoromethylation of p-Anisaldehyde
SyntheticPage 550
DOI:
Submitted: March 29, 2012, published: April 6, 2012
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
A contribution from
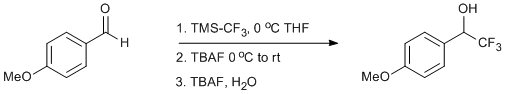
Chemicals
Procedure
Author Comments
Data
1H NMR (CDCl3, 400 MHz) d ppm 3.24 - 3.37 (m, 1 H) 3.80 (s, 3 H) 4.91 (q, J=6.85 Hz, 1 H) 6.92 (d, J=8.80 Hz, 2 H) 7.37 (d, J=8.80 Hz, 2 H) 13C NMR (CDCl3, 101 MHz) d ppm 55.51 (CH3) 72.60 (q, JC-C-F = 31.50 Hz, CH) 114.28 (CH) 120.44 – 128.84 (q, JC-F = 281.70 Hz, CF3) 126.54 (d, JC-C-C-F = 1.47 Hz, CH) 129.07 (C) 160.60 (C) 19F NMR (CDCl3, 377 MHz) d ppm -78.61 (d, J = 6.81 Hz) GC-MS (EI) 206 ([M]+, 37%), 137 (100%), 109 (27%), 94 (28%), 77 (25%), 69 (4%).
Lead Reference
Kelly, C. B.; Colthart, A. M.; Constant, B.D.; Corning, S.R.; Dubois, L. N. E.; Genovese, J. T.; Radziewicz, J. L.; Sletten, E. M.; Whitaker, K. R.; Tilley, J. J. Org. Lett. 2011, 13, 1646.
Other References
Krishnamurti, R.; Bellew, D. R.; Prakash, G. K. S. J. Org. Chem. 1991, 56, 984.
Keywords
addition, alcohols, aldehydes, C-C bond formation, ketones, nucleophilic, organofluorine, Ruppert-Prakash reagent, trifluoromethylation