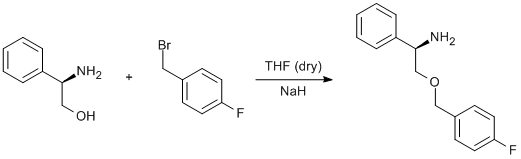
Williamson etherification of a bromomethylfluorobenzene and phenylglycinol
SyntheticPage 674
DOI:
Submitted: July 29, 2013, published: July 30, 2013
Authors
Alan D. Faulkner (a.d.faulkner@warwick.ac.uk)
Daniel H. Simpson (D.H.Simpson@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
Chemicals
(R)-phenylglycinol (prepared in house, see page 275)
THF (distilled over potassium)
1-(bromomethyl)-4-fluorobenzene (Aldrich)
THF (distilled over potassium)
1-(bromomethyl)-4-fluorobenzene (Aldrich)
Procedure
(R)-phenylglycinol (1 g, 7.3 mmol, 1.0 eq) was dissolved in dry THF (15 mL) and was added dropwise to a stirred suspension of NaH (0.36 g, 14.9 mmol, 2.05 eq) in dry THF (10 mL) under an inert atmosphere. The solution was stirred for 1 h at ambient temperature. 1-(bromomethyl)-4-fluorobenzene (0.96 mL, 1.46 g, 7.7 mmol, 1.05 eq) was added drop-wise over 5 min and the solution was stirred for 1 h at ambient temperature. At this point, the solution was heated to reflux (65 °C) under partial vacuum for 3 h before cooling to ambient temperature. This was followed by the addition of brine (40 mL). The product was extracted with diethyl ether(4 x 60 mL), dried over sodium sulfate and the solvent removed to give a pale yellow oil. This crude product was purified by two Kügelrohr distillations; first at 125 °C (under high vacuum) to remove unreacted starting material, and the second at 185 °C (under high vacuum) to give the product, a faint yellow oil.
Yield: 0.386 g (22%)
Yield: 0.386 g (22%)
Author Comments
Procedure was carried out under an inert atmosphere of argon by using a dual manifold vacuum/argon line and standard Schlenk techniques. There is a risk of racemisation of this kind of compound on heating, but our studies on diastereomer formation (see http://dx.doi.org/10.1039/c2dt12378a) indicate that under the conditions specified this is not a problem.
Data
1H NMR (300 MHz, 298 K, CD3CN): δH 7.24 (7H, m, Ar), 6.93 (2H, m, Ar), 4.42 (2H, s, Ar-CH2), 4.13 (1H, dd, 2JHH=3.72 Hz, CH2), 3.49 (1H, dd, 2JHH= 9.05 Hz, 3JHH= 3.72 Hz, CH2), 3.35 (1H, t, 3JHH = 9.04 Hz, CH), 1.65 (2H, s, NH2).
19F NMR (300 MHz, 298 K, CD3CN): δF - 115.39 (s).
MS (ESI) m/z 109.3 [F-C6H4-CH2]+, 246.1 [M+H]1, 268.1 [M+Na]+
19F NMR (300 MHz, 298 K, CD3CN): δF - 115.39 (s).
MS (ESI) m/z 109.3 [F-C6H4-CH2]+, 246.1 [M+H]1, 268.1 [M+Na]+
IR ν cm-1 1603 (w), 1509 (m), 1453 (w), 1355 (w), 1220 (m), 1156 (w), 1087 (m), 1015 (w), 823 (m), 756 (m) 700 (s)
Lead Reference
Bream, R. N.; Ley, S. V.; McDermott, B.; Procopiou, P. A. Journal of the Chemical Society-Perkin Transactions 1 2002, 2237-2242.
http://dx.doi.org/10.1039/b207068p
S. E. Howson, N. P. Chmel, G. J. Clarkson, R. J. Deeth, D. H. Simpson and P. Scott, Dalton Trans., 2012, 41, 4477-4483.
Jahn-Teller effects on π-stacking and stereoselectivity in the phenylethaniminopyridine tris-chelates Cu(NN')32+ http://dx.doi.org/10.1039/c2dt12378a
http://dx.doi.org/10.1039/b207068p
S. E. Howson, N. P. Chmel, G. J. Clarkson, R. J. Deeth, D. H. Simpson and P. Scott, Dalton Trans., 2012, 41, 4477-4483.
Jahn-Teller effects on π-stacking and stereoselectivity in the phenylethaniminopyridine tris-chelates Cu(NN')32+ http://dx.doi.org/10.1039/c2dt12378a
Other References
Evans, D. A.; Peterson, G. S.; Johnson, J. S.; Barnes, D. M.; Campos, K. R.; Woerpel, K. A., J. Org. Chem., 1998, 63, 4541-4544.
http://dx.doi.org/10.1021/jo980296f
http://dx.doi.org/10.1021/jo980296f
Keywords
addition, alcohols, amines, aromatics/arenes, nucleophilic, phenyl glycinol, william etherification