Reduction of phenylglycine
SyntheticPage 275
DOI:
Submitted: June 3, 2008, published: June 3, 2008
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
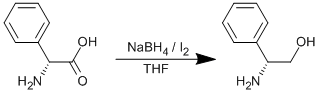
Chemicals
NaBH4 (Fisher Scientific),
Iodine (Sigma Aldrich),
Phenylglycine (Sigma Aldrich),
KOH (Fisher Scientific),
THF (distilled over potassium).
Iodine (Sigma Aldrich),
Phenylglycine (Sigma Aldrich),
KOH (Fisher Scientific),
THF (distilled over potassium).
Procedure
Sodium borohydride (31.21 g, 0.825 mol) in a dry three-necked round-bottom flask was put under Argon and dry THF (400 ml) was added. The solution was stirred whilst phenylglycine (50.00 g, 0.33 mol) was added as a solid in one portion. The flask was cooled to 0oC using an ice-water bath and fitted with a reflux condenser. Iodine (83.75 g, 0.33 mol) in dry THF (100 ml) was added dropwise to the solution. The reaction was then stirred at ambient temperature for 2 h, before heating to reflux (80oC) overnight. Methanol (~400 ml) was added slowly until the solution became clear. All solvent was then removed on the rotary evaporator to leave a white paste, which was then dissolved in aqueous 20% potassium hydroxide solution (600 ml) and stirred at ambient temperature overnight. The product was extracted into dichloromethane (5 x 250 ml), dried over sodium sulphate and the solvent removed to leave the crude product (crude yield = 48.00 g). The product was recrystallised from hot toluene. Yield = 23.33 g, 0.17 mol, 52%.
Author Comments
Adapted from prep. for (S)-tert-leucinol.
Data
1H NMR (400 MHz, 298 K, CDCl3) 7.30-7.18 (5H, m, Ph), 3.97 (1H, dd, 3JHH = 4 Hz, 8 Hz), 3.66 (1H, dd, 2JHH = 11 Hz, 3JHH = 4 Hz, CH2), 3.48 (1H, dd, 2JHH = 11 Hz , 3JHH = 8 Hz, CH2), 2.05 (2H, s, NH2).
Lead Reference
Evans, Peterson, Johnson, Barnes, Campos and Woerpel, J. Org. Chem., 1998, 63, 4541.
Keywords
alcohols, amines, amino acids, amino-alcohol, reduction
Comments
This looks like an excellent procedure and similar to one we reported in SynLett for direct conversion to the N-Boc derivative (Maw, Thirsk, Toujas, Vaultier, Michel; Whiting, Synlett 2004, 1883). In preparing an Org. Syn. procedure (in press), we found racemisation occurred under the reduction conditions very readily, so our recommendation is to check the e.e. after this reduction and stick to the procedure. Some further considerations are in the about to appear Org Syn paper.
From Andy Whiting (9/6/08)
By Andy Whiting on June 9, 2008
Optical Purity
Optical Rotation measured = -25.99 degrees
Optical Rotation reference = -25.8 degrees (Tetrahedron Letters, 1989, 30, 35, 4673-4676).
Conditions: 589 nm, conc = 6.60 g/100 ml, T = 20C, solvent = methanol.
To avoid racemisation, do not distill the product to purify - we have found this causes the product to racemise to an enantiomeric excess of ~40%.
By Suzanne Howson on May 11, 2010
Whiting paper now available
Andy's excellent Org Syn paper is here: http://www.orgsyn.org/orgsyn/pdfs/v85p0219.pdf
By Peter Scott on May 11, 2010
Method 2 (This is a simplier prep but not so good for a large scale reaction >10g)
Lithium aluminium hydride (4.90 g, 129 mmol, 1.95 eq.) was suspended in dry THF (200 ml) under argon at 0°C. Solid (R)-2-phenylglycine (10.00 g, 66.15 mmol, 1.00 eq.) was added in small portions. The mixture was stirred at 0°C for 1 h, then slowly heated to reflux (80°C) overnight. A saturated potassium carbonate solution (75 ml) was added very slowly to the mixture, which was cooled in an ice/water bath. The mixture was filtered and the solvents were removed from the filtrate under reduced pressure. The crude yellow solid was recrystallised from hot toluene to yield a white crystalline solid. Yield 5.74 g (63 %).
By Suzanne Howson on May 19, 2010
Recrystallisation
To maximise product recovery during recrystallise, we have found that a scale of 2 ml of hot toluene for every 1 g of crude product is optimal.
By Suzanne Howson on June 29, 2010