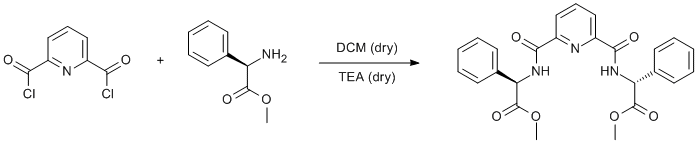
Amidation of a pyridine acylchloride with phenylglycine methyl ester
SyntheticPage 638
DOI:
Submitted: July 19, 2013, published: July 22, 2013
Authors
Nikola P. Chmel (n.chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
Chemicals
Dichloromethane (distilled over potassium)
Triethylamine (distilled over calcium hydride)
Pyridine-2,6-dicarbonyl dichloride (Prepared in house, see page 633)
Procedure
(R)-2-Phenylglycine methyl ester hydrochloride (4.03 g, 20 mmol) was dissolved in dry DCM (40 ml) under argon. Dry TEA (8.4 ml, 60 mmol) was added, and the resulting mixture was filtered into a solution of pyridine-2,6-dicarbonyl chloride (2.04 g, 10 mmol) in dry DCM (40 ml). The reaction was stirred overnight. The resulting mixture was washed with water (3 × 40 ml) and dried over sodium sulphate. The solvents were removed under reduced pressure to obtain yellow oil. Recrystallisation from DCM/pentane yielded white crystalline solid. Yield 2.3 g (49%).
Author Comments
Data
Elemental analysis found (calculated for C25H23N3O6·CH2Cl2) %: C 60.51 (60.78), H 4.77 (4.80), N 8.43 (8.34)
1H NMR (400 MHz, 298K, CDCl3) δH 8.81 (d, 2H, 3JHH = 6.6 Hz, NH2), 8.31 (d, 2H, 3JHH = 7.7 Hz, pyr), 8.02 (t, 1H, 3JHH = 7.7 Hz, pyr), 7.52 (d, 4H 3JHH = 6.8 Hz, Ph), 7.46 – 7.30 (m, 6H, Ph), 5.75 (d, 2H, 3JHH = 7.0 Hz, CH), 5.29 (s, >1H, DCM), 3.79 (s, 6H, OCH3).
13C NMR (100 MHz, 298K, CDCl3) δC 171.02 (CONH), 162.78 (COO), 148.40 (Ph), 139.27, 136.36 (pyr), 129.25, 128.81, 127.38 (Ph), 125.45 (pyr), 56.87 (CH), 53.10 (OCH3).
MS (ESI+) m/z 462.1 ([M+H]+), 484.1 9[M+Na]+); (ESI-) m/z 460.1 ([M-H]-)
IR (cm-1) ν 3379, 2957, 1735, 1677, 1587, 1569, 1505, 1437, 1355, 1326, 1257, 1215, 1195, 1173, 1100, 1072, 998, 945, 926, 859, 842, 788, 781, 754, 732, 695, 671
Lead Reference
Keywords
acyl chloride, addition, alcohols, amides, amines, aromatics/arenes, elimination, ethers