Chlorination of a dicarboxylic acid
SyntheticPage 633
DOI:
Submitted: July 18, 2013, published: July 19, 2013
Authors
Jan Becker (j.m.becker@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
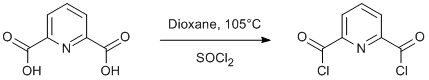
Chemicals
Thionyl chloride (Aldrich)
Dioxane (Fischer Scientific)
Procedure
Pyridine-2,6-dicarboxylic acid (18.3 g, 0.11 mol) and thionyl chloride (19 ml) were dissolved in dioxane (50 ml) and heated to reflux for 3 h at 105°C. The solvent and excess thionyl chloride were removed under reduced pressure. The residue was distilled under vacuum (180-190°C, 4x10-3 mbar). Yield 15.9 g (71%).
Author Comments
Data
Elemental Analysis found (Calculated for C6H4S4) % C 41.38 (41.21), H 1.65 (1.48), N 6.90 (6.86), Cl 31.18 (34.75).
1H NMR (400 MHz, 298 K, CDCl3) δH 8.16 (t, 3H, JHH=8Hz, Ph-H), 8.36 (d, 2H, JHH=8Hz, Ph-H)
13C NMR (100 MHz, 298 K, CDCl3) δC 129.0, 139.4, 149.2, 169.4
MS (ESI+) m/z 167.9 ([M-Cl]-)
IR (cm-1) ν 2744, 2599, 2555, 2453, 1747, 1608, 1576, 1405, 1242, 1199, 1157, 995, 954, 864, 828, 733, 706, 667
Lead Reference
J. P. Leonard, P. Jensen, T. McCabe, J. E. O'Brien, R. D. Peacock, P. E. Kruger and T. Gunnlaugsson, J. Am. Chem. Soc., 2007, 129, 10986-10987. http://dx.doi.org/10.1021/ja073049e
Keywords
acyl chloride, addition, aromatics/arenes, carboxylic acids, elimination, pyridine