Reduction of nitroarene to aniline using tin chloride and acetic acid
SyntheticPage 625
DOI:
Submitted: July 15, 2013, published: July 15, 2013
Authors
Max Hammond (max@flipstorm.net)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
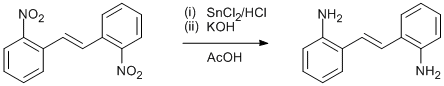
Chemicals
2,2'-dinitrostilbene (Prepared in house, see page 622)
Tin(II)chloride (Aldrich)
Glacial acetic acid (Aldrich)
Conc. HCl
KOH
Tin(II)chloride (Aldrich)
Glacial acetic acid (Aldrich)
Conc. HCl
KOH
Procedure
To SnCl2 (15.4 g, 81.9 mmol) suspended in glacial acetic acid (40 ml) was added concentrated HCl until all SnCl2 had dissolved (~20 ml). To this was added 2,2’-DNS (1.34 g, 4.96 mmol). The mixture was stirred for 15 minutes at room temperature, and then gradually warmed to 70°C. The mixture was stirred at that temperature for 1 h, and then allowed to cool to room temperature. A yellow precipitate (2,2-DAS.2(HCl)) was recovered by filtration, and washed with glacial acetic acid. This was then dissolved in hot water, and basified with KOH, causing a flocculent yellow precipitate to form. After cooling, the precipitate was extracted with Et2O, and dried over MgSO4. The solvent was removed in vacuo, and the yellow solid remaining recrystallized from toluene, leaving 2,2’-diaminostilbene as bright yellow flake-like crystals (0.80 g, 77%).
Author Comments
Conducted several times without problems.
Data
1H NMR 400 MHz (CDCl3): δ ppm 7.40 (dd, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 7.12 (td, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 7.03 (s, 2H, CH=CH), 6.81 (td, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 6.72 (dd, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 5-2.5 (v. br, ~6H, NH3)
13C{1H} NMR 100 MHz (CDCl3): δ ppm 143.9, 128.7, 127.2, 124.1, 119.2, 116.2 (Ar) 125.9 (C=C)
EA: found (calc) C: 79.96 (79.97), H: 6.72 (6.71), N: 13.38 (13.32)
MS (EI+): m/z 120 (M+)
Lead Reference
J. Thiele and O. Dimroth, Ber, 1895, 28, 1411-1414. 10.1002/cber.18950280238
Keywords
alkenes, amine, aromatics/arenes, nitro, reduction, stilbene