Oxidative coupling of 2-nitrobenzyl chloride
SyntheticPage 622
DOI:
Submitted: July 12, 2013, published: July 15, 2013
Authors
Max Hammond (max@flipstorm.net)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
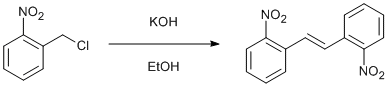
Chemicals
2-nitrobenzyl chloride (Aldrich)
Potassium hydroxide
Ethanol (Fischer Scientific)
Potassium hydroxide
Ethanol (Fischer Scientific)
Procedure
To 2-nitrobenzyl chloride (5.05 g, 29.43 mmol) in EtOH (20 ml) was added slowly KOH (8.26 g, 148 mmol) in warm EtOH (50 ml) with vigorous stirring. The mixture became warm. The mixture was stirred overnight, and a yellow precipitate was recovered by filtration, washed with EtOH, and recrystallized from ethyl acetate, yielding 2,2’-dinitrostilbene as needle like yellow crystals (1.39 g, 17.5%).
Author Comments
Low yield in this reaction, but good enough to take forward to next reduction of NO2 step
Data
1H NMR 400 MHz (CDCl3): δ ppm 8.04 (dd, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 7.81 (dd, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 7.67 (td, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH), 6.57 (s, 2H, CH=CH), 7.48 (td, 4JHH = 1 Hz, 3JHH = 8 Hz, 2H, ArH)
13C{1H} NMR 100 MHz (CDCl3): δ ppm 147.9, 133.7, 132.6, 129.1, 128.9 124.9 (Ar) 130.0 (C=C)
EA: found (calc) C: 62.12 (62.22), H: 3.69 (3.73), N: 10.21 (10.37)
MS (EI+): m/z 270 (M+)
Lead Reference
A. Blanc and C. G. Bochet, J. Org. Chem., 2003, 68, 1138-1141. http://dx.doi.org/10.1021/jo026347x
Keywords
addition, alkenes, benzyl, chloride, elimination, nitro