Protection of phenyl glycinol using phthalic anhydride
SyntheticPage 609
DOI:
Submitted: July 10, 2013, published: July 12, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
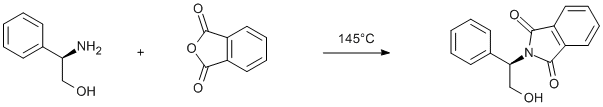
Chemicals
Phthalic anhydride (Aldrich)
Dichloromethane (Fischer Scientific)
Procedure
Solid (R)-phenylglycinol (2.0 g, 14.5 mmol) and phthalic anhydride (2.16 g, 14.5 mmol) were heated with stirring to 145°C for 4 h. The obtained yellow oil was dissolved in DCM (50 ml) and the solution was dried over anhydrous sodium sulphate. The solvent was then removed under reduced pressure and the product was used in the subsequent step without further purification. Yield 3.7 g (95%).
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 7.83 – 7.74 (2H, m, Pht), 7.70 – 7.60 (2H, m, Pht), 7.49 (2H, d, 3JHH = 7.4 Hz, Ph), 7.41 – 7.23 (3H, m, Ph), 5.51 (1H, dd, 3JHH = 8.9 Hz, 3JHH = 5.0 Hz, CH), 4.76 – 4.66 (1H, m, CH2), 4.24 (1H, dd, 3JHH = 11.4 Hz, 4JHH = 4.9 Hz, CH2), 3.47 (1H, s, OH).
13C NMR (100 MHz, 298 K, CDCl3) δC 168.89 (C=O), 136.88, 134.08 (Pht), 131.72, 128.70, 128.13, 127.97 (Ph), 123.31 (Pht), 61.98 (CH2), 57.46 (CH).
MS (ESI+) m/z 290.1 ([M+Na]+)
IR (cm-1) ν 3457, 1772, 1700, 1611, 1585, 1495, 1467, 1388, 1358, 1332, 1288, 1266, 1185, 1172, 1120, 1065, 1040, 1013, 999, 962, 919, 877, 838, 793, 765, 719, 698.
Lead Reference
Keywords
addition, alcohols, amines, phenyl glycinol, phthalic anhydride