Williamson etherification using a hydroxypyridine
SyntheticPage 555
DOI:
Submitted: April 10, 2012, published: April 16, 2012
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
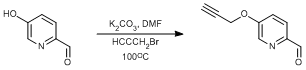
Chemicals
Potassium carbonate (Fisher Scientific)
5-(Hydroxy)picolinaldehyde (SyntheticPage 549)
Dimethylformamide - DMF (Fisher Scientific)
Propargyl bromide - 80% in toluene (Sigma-Aldrich)
Chloroform (Fisher Scientific)
Procedure
Potassium carbonate (0.59 g, 4.26 mmol, 1.05 eq.) was added to a solution of 5-(hydroxy)picolinaldehyde (0.50 g, 4.06 mmol, 1.0 eq.) in DMF (15 ml). Propargyl bromide (80% in toluene, 0.475 ml, 4.26 mmol, 1.05 eq.) was added to the reaction via syringe. The round bottomed flask was fitted with a condenser and the solution was stirred at 100°C for 4 h before allowing to cool to ambient with stirring for an additional 1 h. The solvent was then removed under reduced pressure. The crude solid was then taken up in chloroform (150 ml) and filtered. This process was repeated a further two times (2 × 40 ml). The chloroform was then removed from the filtrate under reduced pressure to leave an orange solid, which was dried overnight in vacuo. Yield = 0.32 g, 1.99 mmol, 49%.
Author Comments
Data
1H NMR (400 MHz, 298 K, DMSO) δH 9.90 (1H, s, C=O), 8.53 (1H, d, 4JHH = 3.0 Hz, Py), 7.97 (1H, d, 3JHH = 8.5 Hz, Py), 7.64 (1H, dd, 3JHH = 8.5 Hz, 4JHH = 3.0 Hz, Py), 5.05 (2H, d, 4JHH = 2.5 Hz, CH2-C≡C), 3.71 (1H, t, 4JHH = 2.5 Hz, C≡CH).
13C{1H} NMR (100 MHz, 298K, DMSO) δC 191.9 (C=O), 156.7 (Py), 146.1 (Py), 138.8 (Py), 123.3 (Py), 121.7 (Py), 79.4 (C≡CH), 78.9 (C≡CH), 56.3 (CH2).
MS (ESI) 162.2 [M+H]+, 184.1 [M+Na]+.
IR ν cm-1: 3213 w, 2127 w, 1692 s, 1569 s, 1490 w, 1474 w, 1379 w, 1308 m, 1282 w, 1259 s, 1203 s, 1132 m, 1006 s, 975 m, 916 w, 835 s, 800 s, 762 m, 732 m, 694 s, 659 s.
Elemental Analysis found (Calculated for C9H7NO2) % C 66.85 (67.07), H 4.02 (4.38), N 8.52 (8.69).
Keywords
addition, alcohols, alkyl/alkenyl/aryl halides, alkynes, aromatics/arenes, etherification, ethers, nucleophilic, substitution, Williamson