Oxidation of 6-(Hydroxymethyl)pyridin-3-ol
SyntheticPage 549
DOI:
Submitted: March 26, 2012, published: April 6, 2012
Authors
Rebecca Kaner (r.a.kaner@warwick.ac.uk)
A contribution from
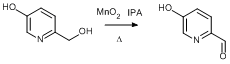
Chemicals
Activated Manganese (IV) Oxide - Sigma-Aldrich
Isopropanol (AR grade) - Sigma-Aldrich
Celite - Sigma-Aldrich
Water - Distilled
Procedure
6-(Hydroxymethyl)pyridin-3-ol (6.74 g, 53.86 mmol, 1.0 eq.) was dissolved in isopropanol (200 ml) to give a brown solution. Activated manganese(IV) oxide (11.71 g, 86.94 mmol, 2.5 eq.) was then added as a solid. The black mixture was stirred at reflux (100°C) for 4 h and then stirred at ambient temperature for a further 18 h. The solution was filtered through celite and the remaining MnO2 was washed with isopropanol (5 × 150 ml). The solvent was removed from the filtrate under reduced pressure to leave a yellow-brown solid. The solid was recrystallised from hot water (~ 20 ml) to leave the pure product as brown crystals. Yield = 3.99 g, 32.41 mmol, 60%.
Author Comments
Data
1H NMR (400 MHz, 298 K, DMSO) δH 11.11 (1H, br s, OH), 9.83 (1H, s, HC=O), 8.31 (1H, d, 4JHH = 2.5 Hz, Py), 7.84 (1H, d, 3JHH = 8.5 Hz, Py), 7.33 (1H, dd, 3JHH = 8.5 Hz, 4JHH = 2.5 Hz, Py).
13C{1H} NMR (100 MHz, 298K, DMSO) δC 191.8 (C=O), 157.9 (Py), 144.7 (Py), 138.7 (Py), 123.6 (Py), 122.3 (Py).
MS (ESI) m/z 124.3 [M+H]+, 146.2 [M+Na]+, 122.0 [M-H]-.
IR v cm-1 2506 w, 1694 m, 1597 w, 1567 s, 1471 w, 1311 m, 1273 m, 1209 s, 1115 s, 1024 m, 911 m, 871 m, 847 s, 790 s, 730 m, 660 s.
Elemental Analysis found (Calculated for C6H5NO2) % C 58.47 (58.54), H 4.07 (4.09), N 11.22 (11.38).
Lead Reference
Keywords
aldehydes, heterocyclic compounds, oxidation