Hydrolysis of the ester 6-{(Acetyloxy)methyl}pyridin-3-yl acetate
SyntheticPage 548
DOI:
Submitted: March 26, 2012, published: April 6, 2012
Authors
Rebecca Kaner (r.a.kaner@warwick.ac.uk)
A contribution from
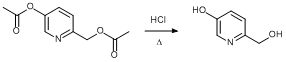
Chemicals
Hydrochloric acid (36%)
Procedure
6-{(Acetyloxy)methyl}pyridin-3-yl acetate (36.59 g, 174.90 mmol) was dissolved in concentrated hydrochloric acid (36%, 400 ml). The black solution was stirred at reflux (110°C) for 24 h. After cooling to ambient temperature, the solvent was removed under reduced pressure until around 50 ml remained. An aqueous solution of sodium hydroxide (1 M) was added dropwise to neutralise the solution (pH 7.0). All solvent was then removed under reduced pressure and the crude brown-grey solid was dried overnight in vacuo at 50°C. The crude solid was then stirred in boiling acetonitrile (250 ml) and filtered hot. This process was repeated a further two times (2 × 250 ml). The yellow-pale brown filtrates were combined and the acetonitrile was removed under reduced pressure to leave the product as a yellow solid, which was dried overnight in vacuo. Yield = 15.81 g, 126.35 mmol, 72%.
Author Comments
Data
1H NMR (400 MHz, 298 K, DMSO) δH 9.72 (1H, br s, Py-OH), 8.02 (1H, d, 4JHH = 3.0 Hz, Py), 7.26 (1H, d, 3JHH = 8.5 Hz, Py), 7.15 (1H, dd, 3JHH = 8.5 Hz, 4JHH = 3.0 Hz, Py), 5.19 (1H, br s, CH2OH), 4.44 (1H, s, CH2).
13C{1H} NMR (100 MHz, 298K, DMSO) δC 152.2 (Py), 152.0 (Py), 136.3 (Py), 122.5 (Py), 121.0 (Py), 63.9 (CH2).
MS (ESI) m/z 126.1 [M+H]+, 148.1 [M+Na]+, 124.1 [M-H]-.
IR v cm-1 3439 w, 2407 w, 1765 w, 1570 m, 1483 m, 1461 m, 1445 m, 1335 m, 1270 s, 1209 s, 1127 m, 1117 m, 1072 s, 1027 m, 893 m, 858 m, 830 s, 760 m, 714 m, 656 s.
Elemental Analysis found (Calculated for C6H7NO2) % C 57.73 (57.59), H 5.51 (5.64), N 10.87 (11.19).
Lead Reference
Keywords
alcohols, heterocyclic compounds, hydrolysis