Complexation of a pyridyldiimine with iron(II) chloride
SyntheticPage 437
DOI:
Submitted: July 6, 2010, published: July 9, 2010
Authors
Giles Theaker (g.w.theaker@warwick.ac.uk)
A contribution from
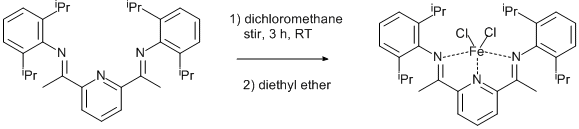
Chemicals
Fe4Cl8.THF6 (doi:10.1016/S0020-1693(00)85366-9)
dichloromethane (Dried by heating to reflux for 72 h over CaH2 then distilling)
diethyl ether (Dried by heating to reflux for 72 h over Na/K alloy then distilling, although other drying agents probably also adequate)
Procedure
2,6-bis[1-(2-isopropylphenylimino)ethyl]pyridine page 436 (0.50 g, 1.04 mmol) and Fe4Cl8.THF6 (0.24 g, 0.52 mmol, 0.5 eq.) were charged into a Schlenk vessel under argon. DCM (30 ml) was added and the reaction mixture was stirred at room temperature for 3 h, after which time the solution had become dark blue. The solution was filtered via cannula into a clean Schlenk vessel, and concentrated to ca 20 ml. Diethyl ether (60 ml) was added, and a blue precipitate formed. This was collected by filtration, washed with diethyl ether (20 ml) and dried in vacuo. (0.51 g, 82 %).
Author Comments
Data
Lead Reference
Other References
B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049-4050 doi:10.1021/ja9802100
Keywords
amines, heterocyclic compounds, Iron, organometallics, pyridyldiimine, substitution, transition metal catalysed