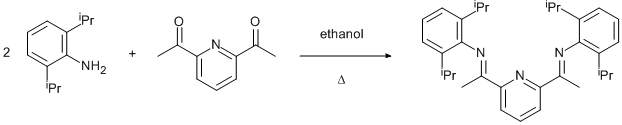
Schiff base formation by condensation of a diketone with amine
SyntheticPage 436
DOI:
Submitted: July 6, 2010, published: July 9, 2010
Authors
Giles Theaker (g.w.theaker@warwick.ac.uk)
A contribution from
Chemicals
2,6-diisopropylaniline, 98% (Sigma-Aldrich)
glacial acetic acid
ethanol
Procedure
2,6-diacetylpyridine (1.50 g, 9.19 mmol) was dissolved in ethanol (25 ml) in a 50 ml round bottom flask. To this was added 2,6-diisopropylaniline (3.50 ml, 3.25 g, 18.38 mmol, 2 eq.) and 2 drops of glacial acetic acid. The solution was heated to reflux for ca 20 h. After this time, a crude sample was taken and shown by NMR to be monosubstituted 2,6-diacetylpyridine. A further 1 ml of 2,6-diisopropylaniline was added, and reflux continued for a further 24 h. Upon cooling light yellow crystals were formed. These were collected by filtration and recrystallised from ethanol. (1.84 g, 41 %).
Author Comments
These compounds are used as ligands for Brookhart/Gibson olefin polymerization catalysts; see page 437.
Data
1H NMR (400 MHz, CDCl3): δ 8.41 (2H, d, 3J = 8 Hz, Ar), 7.86 (1H, t, 3J = 8 Hz, Ar), 7.11 (4H, d, 3J = 7 Hz, Ar), 7.03 (2H, t, 3J = 7 Hz, Ar), 2.70 (4H, sept., 3J = 7 Hz, CHMe2), 2.20 (6H, s, NCMe), 1.19 (24H, d, 3J = 7 Hz, CHMe2).
13C NMR (100 MHz, CDCl3): δ 166.9 (CN), 155.2, 146.5 (Ar q), 136.9 (Ar), 135.8 (Ar q) 123.6, 123.0, 122.2 (Ar), 28.3 (CMe2), 23.2 (Me), 22.9 (CMe2).
Lead Reference
B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049-4050 doi:10.1021/ja9802100
Keywords
amination, amines, Condensation, heterocyclic compounds, imines, ketones, Schiff base