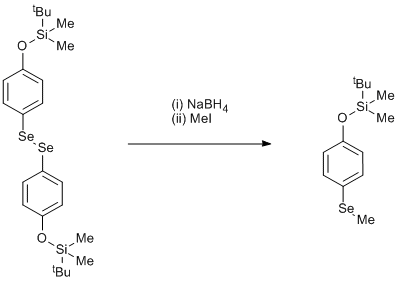
Borohydride cleavage of a diselenide followed by alkylation
SyntheticPage 431
DOI:
Submitted: July 4, 2010, published: July 9, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
Chemicals
Procedure
The unpurified bis (4-tert-butyldimethylsilyloxyphenyl) diselenide (20.0 g 0.035 mol) was diluted with ethanol (200 cm3) and with magnetic stirring under nitrogen, solid NaBH4 (a total of 16.0 g. 0.42 mol) was added (exothermic reaction – up to 55 °C) in portions over 3 h, to give a colourless solution. Excess iodomethane (50 cm3 0.80 mol) was added and the mixture heated at 55 °C for 1 h. Water (250 cm3) was added and the mixture extracted with 1: 1 diethyl ether - 80/100 petroleum ether (3 x 100 cm3). The combined organic phases were washed with water, HCl (1 M), dried (MgSO4), evaporated and the residue distilled to give a colourless oil, b.p. 76-80 ºC/0.01 mm, 8.0 g, 38% yield.
Author Comments
Given the unknown purity of the diselenide starting material, the 38% figure for the yield is probably a minimum value.
tert-Butyl-dimethyl-(4-n-propylselanyl-phenoxy)-silane was prepared in a similar manner, using 1-bromopropane in place of iodomethane, giving a colourless oil, b.p. 82-88 ºC/0.01 mm,
δH (CDCl3, 60 MHz) 7.09d (2 H), 6.46d (2 H), 2.99t (2 H), 1.55sex (2 H), 0.96s + t (12 H), 0.19s (6 H)
Data
Lead Reference
Other References
Keywords
alkylation, reduction