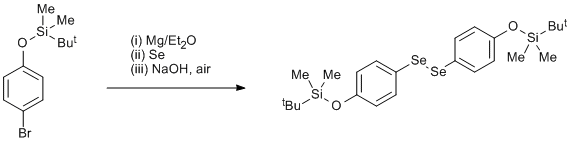
Selenation of a Grignard reagent
SyntheticPage 430
DOI:
Submitted: July 4, 2010, published: July 5, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
Chemicals
Procedure
A mixture of (4-bromophenoxy)-tert-butyldimethylsilane (23.8 g, 0.083 mol) and 1,2-dibromoethane (9.0 g, 0.048 mol) in diethyl ether (50 cm3) was added dropwise to a stirred suspension of magnesium metal turnings (7.0 g, 0.29 mol) in diethyl ether (50 cm3) under nitrogen. When the reaction commenced more diethyl ether (100 cm3) was added and the rate of addition regulated to maintain gentle reflux. After the end of the addition the mixture was refluxed for 5 h. After cooling, the solution was decanted from excess magnesium metal. Precipitated selenium (7.0 g, 0.091 mol) added over a period of 15 min. Stirring was continued for 12 h, then, with ice-cooling, HCl (50 cm3, 2 M) was added and the suspension filtered. The phases of the filtrate were separated and the diethyl ether phases evaporated to give a viscous orange oil which was dissolved in ethanol (200 cm3), NaOH (2.0 g) added and air was bubbled through for 24 h. The dark solution was filtered with charcoal and Celite and evaporated to give a deep yellow oil (22.3 g) which was used directly for the next step.
Author Comments
A Grignard reaction with added 1,2-dibromoethane (which ends up as ethylene) to promote a steady reaction and with excess Mg to ensure conversion of all the starting material was used. The product was not purified or characterised and was used directly for further syntheses.
Data
Lead Reference
Other References
Keywords
Grignard, oxidation, selenium