Silyl protection of a phenol
SyntheticPage 429
DOI:
Submitted: July 4, 2010, published: July 8, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
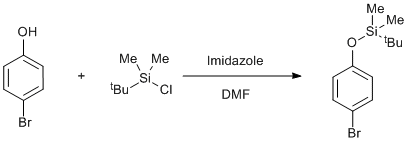
Chemicals
tert-butyl(chloro)dimethylsilane (Aldrich)
4-Bromophenol (Aldrich)
Imidazole (Aldrich)
Dimethylformamide (Aldrich)
Procedure
4-Bromophenol and imidazole were dried in a dessicator over P2O5. tert-Butyl(chloro)dimethylsilane (15.0 g, 0.1 mol) was added to a solution of 4-bromophenol (12.1 g, 0.07 mol) and imidazole (16.5 g, 0.24 mol) in dimethylformamide (50 cm3) and the mixture was magnetically stirred (2 d). Water (5 cm3) and 60/80 petrol (50 cm3) were then added, followed after 1 h by more water (100 cm3). The phases were separated and the aqueous phase extracted twice with 1: 1 60/80 petrol-diethyl ether (100 cm3). The combined organic phases were washed successively with water, aqueous NaOH solution (1 M), water and saturated NaCl solution and dried (MgSO4). The solution was evaporated and the residue distilled to give a colourless oil, 19.8 g (98%), b.p. 67-68 ºC/0.01 mm.
Author Comments
This well documented reaction proceeded without problems. The literature yield was 90%.
Data
δH (CDCl3, 60 MHz), 7.13d (2 H), 6.25d (2H), 0.93s (9 H), 0.16s (6 H).
δC (CDCl3, 100 MHz), 154.78, 132.26, 121.68, 113.60, 25.61, 18.16, -4.50.
Lead Reference
Riley PA, Cooksey CJ, Johnson CI, Land EJ, Latter AM, Ramsden CA., Eur J Cancer, 1997, 33(1), 135-143. PubMed PMID: 9071913. doi: 10.1016/S0959-8049(96)00340-1
Other References
Angle, SR, Louie, MS, J. Org. Chem., 1991, 56(8), 2853-2866. doi: 10.1021/jo00008a049
Keywords
phenol, protection, silylation