Addition of 1,2-ethanedithiol to 2-bromo-1,1-diethoxyethane
SyntheticPage 418
DOI:
Submitted: May 28, 2010, published: June 1, 2010
Authors
Nikola Paul Chmel (n.chmel@warwick.ac.uk)
A contribution from
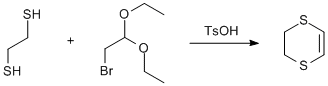
Chemicals
1,2-ethanedithiol
2-bromo-1,1-diethoxyethane
Procedure
Toluene (330 ml) and p-toluenesulfonic acid monohydrate (0.33 g, 1.7 mmol) were heated to reflux in Dean-Stark apparatus under nitrogen for 24 h. The collected water was removed and the Dean-Stark apparatus was replaced with a reflux condenser. 1,2-ethanedithiol (14 ml, 0.167 mol) and 2-bromo-1,1-diethoxyethane (24.5 ml, 0.163 mol) were added and the mixture was heated to reflux for 24 h. The cooled mixture was washed with water (300 ml) and separated. The water layer was back extracted with diethyl ether (3 × 100 ml). The combined organic extracts were washed with brine (3 × 100 ml) and dried twice over anhydrous Na2SO4. The solvents were removed in vacuo and the residue was distilled under vacuum (50°C, 4·10-3 mbar). Yield 6.46 g (34%).
Author Comments
This is an intermediate in the synthesis of BEDT-TSF (BETS).
Data
1H NMR (400 MHz, 298K, CDCl3) δH 6.04 (s, 2H, CH), 3.14 (s, 4H, CH2)
13C NMR (101 MHz, 298K, CDCl3) δC 114.39 (CH), 26.30 (CH2)
Lead Reference
Keywords
addition, alkenes, alkyl/alkenyl/aryl halides, BEDT-TSF, BETS, bisethylenedithiotetraselenafulvalene, elimination, ethers, heterocyclic compounds, nucleophilic, reduction, substitution, sulphides