Reductive coupling of 4,5-ethylenedithio-1,3-diselenol-2-one
SyntheticPage 421
DOI:
Submitted: May 28, 2010, published: June 1, 2010
Authors
Nikola Paul Chmel (n.chmel@warwick.ac.uk)
A contribution from
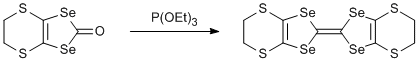
Chemicals
4,5-ethylenedithio-1,3-diselenol-2-one synthesis
triethyl phosphite
toluene (refluxed over potassium, degassed)
Procedure
Triethyl phosphite (10 ml) was added dropwise over 15 min to a solution of 4,5-ethylenedithio-1,3-diselenol-2-one (0.3 g, 0.99 mmol) in dry toluene (30 ml) under argon. The mixture was heated to reflux for 2 h and then allowed to cool. The product was collected by filtration and recrystallised from hot anhydrous carbon disulfide under argon (CAUTION - CS2 has a very low flash point and is highly inflammable). Yield 0.15 g (52%).
Author Comments
BETS is really insoluble - CS2 was the best solvent we found for recrystallisation, it also dissolves sparingly in chloroform.
This procedure has been published in outline by Kato et al. and Courcet et al., to our knowledge however this is the first detailed synthetic procedure for this reaction. The coupling procedure was adapted from Imakubo et al.
Data
Elemental analysis found (calculated for C10H8S4Se4) %: C 21.10 (20.99), H 1.20 (1.41)
1H NMR (400 MHz, 298K, CDCl3) δH 1.56 (s, 8H, CH2)
13C NMR, - no data obtained even on 700MHz and 16k scans - due to very low solubility
Lead Reference
Other References
T. Courcet, I. Malfant, K. Pokhodnia and P. Cassoux, New J. Chem., 1998, 22, 585-589.
T. Imakubo, M. Kibune, H. Yoshino, T. Shirahata and K. Yoza, J. Mater. Chem., 2006, 16, 4110-4116.
Keywords
alkenes, BEDT-TSeF, BEDT-TSF, BETS, heterocyclic compounds