Synthesis of an N-heterocyclic carbene via deprotonation of an imidazolinium salt
SyntheticPage 34
DOI:
Submitted: July 17, 2001, published: July 17, 2001
Authors
Lisa Titcomb (lisa.titcomb@gmail.com)
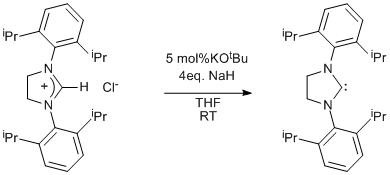
Chemicals
1,3-Bis-(2,6-diisopropylphenyl)imidazolinium Chloride (see Page 31).
KOtBu (Aldrich, sublimed twice before use, 10-5mbar, 160oC).
THF (heated at reflux over potassium for three days then distilled and stored in an ampoule over sieves).
Sodium hydride (purchased dry from Aldrich (95%) and stored in a glove box).
Standard Schlenk line techniques were used for all manipulations involved in the deprotonation of the imidazolinium Chloride and subsequent usage of the carbene (see ref 1).
KOtBu (Aldrich, sublimed twice before use, 10-5mbar, 160oC).
THF (heated at reflux over potassium for three days then distilled and stored in an ampoule over sieves).
Sodium hydride (purchased dry from Aldrich (95%) and stored in a glove box).
Standard Schlenk line techniques were used for all manipulations involved in the deprotonation of the imidazolinium Chloride and subsequent usage of the carbene (see ref 1).
Procedure
1,3-Bis-(2,6-diisopropylphenyl)imidazolinium chloride (28g, 0.66mol) was placed in a Schlenk tube, attached to a double manifold Schlenk line and cycled vacuum/argon three times. It was then evacuated and transferred to a glove box. KOtBu (0.37g, 3.3x10-3mol) and NaH (6.3g, 0.263mol) were added and the tube capped and removed from the glove box. The tube was reattached to the Schlenk line and THF (200 mL) was added. The tube was left open to argon. An oil bubbler was attached to a second tap on the Schlenk line and opened to argon. The mixture was stirred under an oil bubbler pressure of Ar for 17h. The mixture was filtered through Celite on sintered glass and the THF removed to yield a white solid. The solid was dried under vacuum to yield 25.5g of product (99% yield).
Author Comments
This is the last stage in the Arduengo carbene prep. For other stages see Other References #3 below. This deprotonation procedure differs from that described in the lead reference and follows the procedure described in reference 2. The product is obtained in high yield and purity and can be used in further reactions without the need for recrystallisation. However if purification is necessary, washing the solid with cold pentane removes any impurities. The pentane washings can be concentrated and cooled to -50oC to yield more carbene.
Data
1H NMR (D6-Benzene): 1.24 (d, 12H, iPrCH3, 3J(HH) = 6.9 Hz), 1.29 (d, 12H, iPrCH3, 3J(HH) = 6.9 Hz), 3.23 (m, 4H, iPrCH, 3J(HH) = 6.9 Hz), 3.31 (s, 4H, CH2), 7.18 (m, 6H, aryl-CH).
Lead Reference
Arduengo, A. J.; Krafczyk, R.; Schmuter, R. Tetrahedron, 1999, 55, 14523.
Other References
1. Shriver, D. F. The Manipulation of Air Sensitive Compounds, McGraw-Hill, New York, 1989. 2. Arduengo, A. J.; Dias, H. V. R.; Harlow, R. L.; Kline, M. Journal of the American Chemical Society, 1992, 114, 5530-5534. 3. This multi stage synthesis of the Arduengo carbene begins at Page 28 and continues Page 30, Page 31 then ends here.
Keywords
Arduengo carbene, Carbene, deprotonation, imidazol-2-ylidene, imidazolin-2-ylidene, N-heterocyclic, radical