Condensation of an amine with glyoxal
SyntheticPage 28
DOI:
Submitted: July 10, 2001, published: July 10, 2001
Authors
Lisa Titcomb (lisa.titcomb@gmail.com)
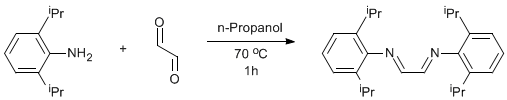
Chemicals
40% aqueous glyoxal (Aldrich)
2,6-diisopropylphenylamine (Aldrich)
n-propanol (Aldrich)
2,6-diisopropylphenylamine (Aldrich)
n-propanol (Aldrich)
Procedure
40 % aqueous glyoxal (14.7g, 0.101mol of glyoxal) in n-propanol (16 mL) and water (40 mL) was added to a solution of 2,6-diisopropylphenylamine (39.6g, 0.224mol) in n-propanol (160 mL). The mixture was heated at 70oC with stirring for 1h. 160 mL of water was added to the mixture. The yellow precipitate formed was collected on a frit and redissolved in the minimum of hot n-propanol. Water (200 mL) was added to reprecipitate the yellow solid. The solid was collected on a frit and dried under vacuum to yield 32.9 g of product (87% yield).
Author Comments
This procedure works well, however prolonged heating can result in lower yields. 2,4,6-trimethylphenylamine has also been used in this condensation reaction.
Data
1H NMR (CDCl3): 1.20 (d, 24H, iPr-CH3, 3J(HH) = 6.9 Hz), 2.91 (sept, 4H, iPr-CH, 3J(HH) = 6.9 Hz), 7.16 (m, 6H, aryl-CH), 8.03 (s, 2H, CH)
Lead Reference
Arduengo, A. J.; Krafczyk, R.; Schmuter, R. Tetrahedron, 1999, 55, 14523.
Other References
For next stage in carbene prep see Page 30
Keywords
addition, condensation, Diimine