Diiodination of 1,4-dimethoxybenzene in methyl alcohol
SyntheticPage 762
DOI:
Submitted: September 9, 2014, published: September 17, 2014
Authors
Nchong Ebai (ebainchong@yahoo.com)
Nicholas Marshall (Nicholas_Marshall@berea.edu)
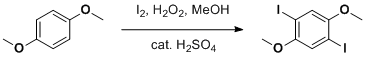
Chemicals
1,4-dimethoxybenzene (Aldrich Chemical Company)
Iodine, pelleted
95% methyl alcohol
Water, deionized
30% hydrogen peroxide (Fisher Scientific)
40% (7.2M) sulfuric acid (diluted from conc. sulfuric acid, Fisher Scientific)Procedure
Author Comments
-
As in our previous work on 1,4-dibromination of these type of compounds in SyntheticPage 753, these compounds are useful intermediates for conjugated polymer synthesis but we are interested in developing more environmentally friendly preps for them.
-
We have found that both common 95% ethyl alcohol as well as 10% aqueous ethyl alcohol can be used as solvents for this reaction. These reactions can be worked up by rotary evaporation of the crude reaction mixture to remove ethanol and water, which requires an efficient pump on the rotovap. We prefer the methanol solvent when running the reaction on a preparative scale (see below). With low concentrations of ethanol in the solvent, we occasionally saw the iodine "climbing" up the condenser and getting stuck due to its insolubility in the condensing vapor; if we observed this, we added a mL or two of 95% ethanol to wash it back down and continue the reaction.
-
We have performed this reaction on a 20 mmol scale in methanol as well. On this reaction scale, microcrystalline product precipitates spontaneously and can be isolated by vacuum filtration followed by washing with cold MeOH to give 3.53 g (45%) fine white crystals. Some product remains in the mother liquor and can be recovered by successive evaporation and crystallization to improve the yield.
-
At the 20 mmol scale, it is particularly important to add the hydrogen peroxide slowly over several minutes to avoid an exotherm. We encountered this problem once when the peroxide was added to the hot reaction mixture in one large portion - we attribute it to an "elephant's toothpaste" type decomposition of the peroxide catalyzed by iodine.
-
We have also used this system to prepare the corresponding 2,5-bis(n-octyloxy) and (n-butyloxy) 1,4-diiodobenzenes. These products do not precipitate out from the reaction mixture but must be isolated with an aqueous workup.
Data
Lead Reference
Other References
Keywords
alkyl/alkenyl/aryl halides, aqueous, aromatics/arenes, carbocyclic compounds, iodination, substitution