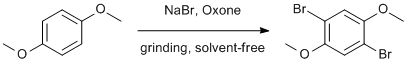
Solventless dibromination of 1,4-dimethoxybenzene
SyntheticPage 753
DOI:
Submitted: July 10, 2014, published: August 12, 2014
Authors
Nicholas Marshall (nicholas_marshall@berea.edu)
A contribution from
Chemicals
1,4-dimethoxybenzene
Sodium bromide
Potassium peroxymonosulfate, triple salt with potassium sulfate and potassium hydrogen sulfate
Water
Sodium bromide
Potassium peroxymonosulfate, triple salt with potassium sulfate and potassium hydrogen sulfate
Water
Procedure
1,4-dimethoxybenzene (0.56 g, 4.0 mmol) was placed in a mortar and pestle and sodium bromide (0.82 g, 8.0 mmol) was added. Oxone (2.45 g, 4.0 mmol) was added and the mixture was ground for approximately 15 min in the mortar until the reaction mixture had attained a uniform waxy texture. The solid was washed with water while grinding in the mortar, and collected on a fritted funnel. The product was recrystallized from 95% ethyl alcohol by transferring the solid to an Erlenmeyer flask and gradually heating with a heat gun while adding ethanol until the solid dissolved entirely. Slow cooling on the bench to room temperature followed by cooling in an ice bath gave white, needle-like crystals, which were collected by Buchner filtration and rinsed with ice-cold ethanol. (0.98g, 83% yield)
Author Comments
- 1,4-dibromo-2,5-dialkoxybenzenes are useful synthons in the synthesis of conjugated polymers, but typical syntheses use halogenated solvents and liquid bromine, which are relatively hazardous and environmentally costly. (See Other References)
- The reagents are placed in a common Coors mortar and pestle and mixing and grinding take place simultaneously.
- Initially, the solid reaction mixture is a homogeneous, free-flowing, entirely white powder; as grinding continues for a few minutes, the reaction mixture develops a tacky or plastic texture. After this, orange-brown streaks begin appearing under the head of the pestle, presumably from the transient presence of elemental bromine. At the end of the reaction, the product will be a waxy solid with the orange color of unreacted bromine.
- The reaction mixture can be readily worked up by the addition of water and further grinding to mix the solid with water, followed by simply pouring off the aqueous wash solution into a vacuum filtration setup. Washing with water yields a pinkish, dense, grainy solid which is easily collected by filtration.
- The reaction should be done in a well-ventilated area since it emits fumes of HBr vapor while the reaction is occurring. After completion, the product has a distinctive sharp smell which is easily distinguished from the sweet "urinal-cake" odor of the starting material.
- This reaction has been efficient in our hands at brominating 1,4-dibutoxybenzene and 1,4-dioctyloxybenzene as well; although the crude product is oily and less convenient to transfer than the methoxy product, it is extremely hydrophobic and cohesive allowing it to be easily removed from the mortar and transferred in one lump to the crystallization step. Filtration is not recommended, although we blotted with a Kimwipe to remove excess water.
Data
1H NMR (CDCl3, δ vs. TMS, 300 MHz): 7.10 (s, 2H) 3.84 (s, 6H). 13C NMR (CDCl3, δ vs. TMS, 75 MHz): 150.6, 117.2, 110.6, 57.1
Lead Reference
H. Firouzabadi, N. Iranpoor, and S. Kazemi, Can. J. Chem., 2009, 87, 1675-1681.
Other References
Ryo Miyakoshi, Kyohei Shimono, Akihiro Yokoyama, and Tsutomu Yokozawa J. Am. Chem. Soc., 2006, 128 (50), 16012-16013.
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes, electrophilic, ethers, green, solid-phase, solventless, substitution