Methanesulfonylation of BOC protected (S)-Alaninol
SyntheticPage 615
DOI:
Submitted: July 11, 2013, published: July 15, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
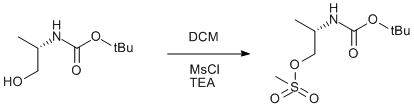
Chemicals
(S)-tert-butyl 1-hydroxypropan-2-ylcarbamate (Prepared in house, see page 614)
Dichloromethane (Fischer Scientific)
Triethylamine (BDH)
Procedure
A solution of methanesulfonyl chloride (0.93 ml, 12 mmol) in DCM (20 ml) was added dropwise over 30 min to a solution of (S)-tert-butyl 1-hydroxypropan-2-ylcarbamate (2.0 g, 11 mmol) and TEA (1.8 ml, 12.5 mmol) in DCM (40 ml). The volatiles were then removed under reduced pressure and the residue was redissolved in ethyl acetate (30 ml) and water (30 ml). The organic layer was washed with an aqueous solution of NaHCO3 (30 ml, 5%), brine (30 ml) and dried over anhydrous Na2SO4. The product was obtained upon removal of the solvent under reduced pressure. Yield 2.1 g (75%).
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 4.61 (1H, br s, NH), 4.21 (1H, br s, CH2), 4.15 (1H, br s, 3JHH = 10.0 Hz, 2JHH = 4.3 Hz, CH2), 4.05 – 3.90 (1H, br s, CH), 3.02 (3H, s, SO3CH3), 1.44 (9H, s, tBu), 1.22 (3H, d, 3JHH = 6.9 Hz, CHCH3).
13C NMR (100 MHz, 298 K, CDCl3) δC 155.22 (C=O), 72.17 (CH2), 45.65 (CH), 37.45 (SO3CH3), 28.45 (C(CH3)3), 17.30 (CHCH3), no quaternary carbon peak found
MS (ESI+) m/z 276.1 ([M+Na]+)
IR (cm-1) ν 3358, 2978, 2937, 1687, 1527, 1463, 1430, 1394, 1368, 1347, 1331, 1301, 1279, 1242, 1159, 1109, 1061, 1031, 996, 972, 941, 927, 900, 850, 812, 779, 743.
Lead Reference
K. Higashiura, H. Morino, H. Matsuura, Y. Toyomaki and K. Ienaga, J. Chem. Soc., Perkin Trans. 1, 1989, 1479-1481.http://dx.doi.org/10.1039/p19890001479
Supplementary Information
Keywords
addition, alcohols, BOC protected, esters, methanesulfonylation