BOC protection of (S)-Alaninol
SyntheticPage 614
DOI:
Submitted: July 11, 2013, published: July 15, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
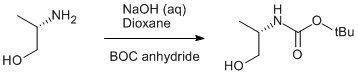
Chemicals
1M NaOH (aq)
BOC-anhydride (Fluka)
Dioxane (Fischer Scientific)
Procedure
(S)-Alaninol (2.0 g, 26.6 mmol) was dissolved in aqueous NaOH solution (50 ml, 1 M) and was cooled in an ice/water bath. A solution of di-tert-butyldicarbonate (6.5 g, 30.0 mmol) in dioxane (30 ml) was added and the mixture was stirred for 3 h. The mixture was then allowed to separate. The aqueous layer was acidified to pH ≈ 8 using a saturated NH4Cl solution and extracted with ethyl acetate (4 × 30 ml). The organic layer and ethyl acetate extracts were combined and the solvents were removed under reduced pressure. The obtained residue was extracted with ethyl acetate/hexane (1:2) mixture, filtered and the solvent were removed under reduced pressure. The obtained product was used in the subsequent step without further purification. Yield 3.3 g (71%).
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 4.78 (1H, s, NH), 3.73 (1H, s, CH), 3.59 (1H, dd, 3JHH = 10.8 Hz, 2JHH = 3.5 Hz, CH2), 3.47 (1H, dd, 3JHH = 10.8 Hz, 2JHH = 6.0 Hz, CH2), 3.06 (1H, s, OH), 1.42 (9H, s, tBu), 1.12 (3H, d, 3JHH = 6.8 Hz, CHCH3).
13C NMR (100 MHz, 298 K, CDCl3) δC 156.31 (C=O), 79.50 (C(CH3)3), 66.54 (CH2), 48.38 (CH), 28.40 (C(CH3)3), 17.33 (CHCH3).
MS (ESI+) m/z 198.2 ([M+Na]+)
MS (ESI-) m/z 174.8 ([M]-)
IR (cm-1) ν 3445, 3340, 2977, 2938, 2878, 1675, 1650, 1531, 1456, 1393, 1363, 1344, 1313, 1277, 1251, 1160, 1102, 1060, 1025, 934, 915, 880, 843, 781, 754.
Lead Reference
A. Benalil, B. Carboni and M. Vaultier, Tetrahedron, 1991, 47, 8177-8194. http://dx.doi.org/10.1016/S0040-4020(01)91013-0
Keywords
addition, Alaninol, alcohols, amines, BOC anhydride, esters, protection