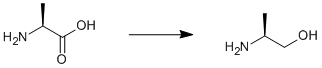
Reduction of L-alanine
SyntheticPage 292
DOI:
Submitted: July 25, 2008, published: July 25, 2008
Authors
Nikola Paul Chmel (n.chmel@warwick.ac.uk)
Chemicals
Lithium aluminium hydride,
THF (distilled over potassium, degassed),
L-alanine
THF (distilled over potassium, degassed),
L-alanine
Procedure
Lithium aluminium hydride (17 g, 0.43 mol) was suspended in dry THF (600 ml) under argon at 273 K. Solid L-Alanine (20 g, 0.22 mol) was added in small portions. The mixture was heated slowly to reflux overnight. A saturated potassium carbonate solution (~100 ml) was added very slowly to the mixture, which was cooled in an ice/water bath. The mixture was filtered and solvents were removed under reduced pressure. The residue was distilled under high vacuum. Yield 12.7 g (77%).
Author Comments
Freshly distilled compound is colorless, after few hours slight yellow coloration occurs.
Data
1H NMR (400 MHz, CDCl3, 298 K) 0.96 (dd, 3H, 2JHH=6Hz, 3JHH=1Hz, CH3), 2.58 (br s, 3H, NH2+OH), 2.87-2.97 (m, 1H, CH), 3.15 (overlapping dd, 1H, 2JHH=8Hz, 3JHH=1Hz, CH2), 3.44 (ddd, 1H, 2JHH=11Hz, 3JHH=4Hz, 3JHH=1Hz, CH2) 13C NMR (100 MHz, CDCl3, 298 K) 19.4 (CH3), 48.2 (CH), 67.8 (CH2)
Lead Reference
Hsiao, Y.; Hegedus, L. S. J. Org. Chem. 1997, 62, 3586-3591.
Keywords
alcohols, amines, amino acids, amino-alcohol, reduction
Comments
Similar method [[[275|Page 275]]] using NaBH~4~/I~2~ doesn't work for alaninol, however it works for most of the other aminoacids.
By Nikola Paul Chmel on July 29, 2008
Do you think this would work for Histidine to Histidinol ?
By Mark Bertinetti on September 17, 2009
I'd try [[275|Page 275]] first. It's only alaninol that ever gave me any problems. I've never used histidine though.
By Nikola Paul Chmel on September 18, 2009