Iodination of BOC protected phenylglycinol
SyntheticPage 612
DOI:
Submitted: July 10, 2013, published: July 15, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
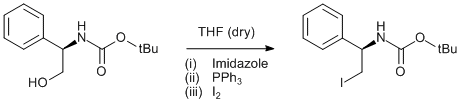
Chemicals
Imidazole (Aldrich)
Triphenylphosphine (Aldrich)
THF (Distilled over potassium)
Iodine (Acros)
Procedure
(R)-tert-butyl 2-hydroxy-1-phenylethylcarbamate (1.0 g, 4.2 mmol) was dissolved in dry THF (50 ml) under argon and cooled in an ice/water bath. Imidazole (1.06 g, 15.6 mmol), triphenylphosphine (2.91 g, 11.1 mmol) and iodine (2.72 g, 10.7 mmol) were added in that order. The resulting mixure was stirred for 1 h at 0°C and then at ambient temperature for 2 d. The reaction was quenched with a saturated solution of sodium thiosulphate (40 ml) and brine (40 ml). The aqueous layer was extracted with DCM (30 ml) and the combined organic layers were dried over anhydrous magnesium suphate. The solvents were removed under reduced pressure and the residue was purified on silica using DCM as eluent. Yield 0.85 g (58%).
Author Comments
Data
Elemental analysis found (calculated for C13H18NO2) %: 45.50 (44.97), H 5.35 (5.23), N 4.01 (4.03).
1H NMR (400 MHz, 298 K, CDCl3) δH 7.40 – 7.21 (5H, m, Ph), 5.04 (1H, s, NH), 4.78 (1H, s br, CH), 3.59 – 3.40 (2H, m, CH2), 1.43 (9H, s, tBu).
13C NMR (100 MHz, 298 K, CDCl3) δC 128.90, 128.17, 126.38 (Ph), 55.46 (CH), 28.48 (CH3), 12.33 (CH2).
MS (ESI+) m/z 370.0 ([M+Na]+)
IR (cm-1) ν 3354, 3009, 2981, 1688, 1521, 1453, 1417, 1392, 1368, 1351, 1333, 1316, 1279, 1247, 1155, 1123, 1079, 1044, 1020, 914, 877, 842, 758, 742, 698, 658.
Lead Reference
Other References
Keywords
aromatics/arenes, esters, Iodination, substitution