BOC protection of phenylglycinol
SyntheticPage 611
DOI:
Submitted: July 10, 2013, published: July 12, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
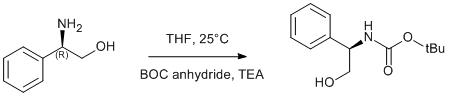
Chemicals
THF (Fischer Scientific)
Triethylamine (BDH)
BOC anhydride (Fluka)
Procedure
(R)-phenylglycinol (1.0 g, 7.3 mmol) was dissolved in THF (10 ml) and cooled in an ice/water bath. A solution of di-tert-butyldicarbonate (1.08 g, 7.7 mmol) in THF (10 ml) and TEA (2.14 ml, 15.4 mmol) were added and the reaction mixture was stirred for 2 h at ambient temperature. The resulting solution was concentrated under reduced pressure to approximately half volume and hexanes were added to initialise crystallisation. The obtained solid was collected by suction filtration and washed with hexane. Yield 1.72 g (99%).
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 7.36 – 7.21 (5H, m, Ph), 5.30 (1H, s br, NH), 4.74 (1H, s br, CH), 3.78 (2H, s br, CH2), 2.62 (1H, s, OH), 1.40 (9H, s, tBu).
13C NMR (100 MHz, 298 K, CDCl3) δC 156.29 (C=O), 139.65, 128.85, 127.79, 126.69 (Ph), 80.10 (C(CH3)3), 66.90 (CH2), 56.95 (CH), 28.46 (C(CH3)3).
MS (ESI+) m/z 260.1 ([M+Na]+)
IR (cm-1) ν 3237, 1670, 1605, 1584, 1547, 1493, 1469, 1454, 1431, 1392, 1367, 1343, 1314, 1283, 1255, 1232, 1159, 1103, 1054, 1027, 918, 866, 842, 760, 701, 663.
Lead Reference
Supplementary Information
Keywords
addition, alcohols, amines, aromatics/arenes, BOC protection, esters