Chlorination of a carboxylic acid
SyntheticPage 517
DOI:
Submitted: October 28, 2011, published: November 27, 2011
Authors
Anish Mistry (a.mistry@warwick.ac.uk)
A contribution from
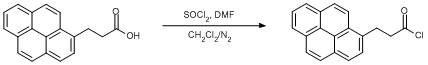
Chemicals
Procedure
3-(1-Pyrenyl)propionic acid (0.1 g, 0.36 mmol) was dissolved in DCM (15 ml) with stirring and placed in an ice bath (0oC) under a dinitrogen atmosphere. Thionyl chloride (0.026 ml, 0.36 mmol) was then added carefully and thereafter DMF (two drops). The reaction was left in the inert atmosphere for an hour at room temperature. The solvent was removed in vacuo and an orange crystalline solid is formed in high yield (96%).
Author Comments
When concentrating solution in vacuo make sure it is in a fume hood due to toxic gases expelled.
Data
δH(400MHz, CDCl3) ppm: 3.46 (2H, t, J 8, 7.5, CH2), 3.81 (2H, t, J 8, 7.5, CH2), 7.92 (1H, d, J 8, aryl), 8.01 - 8.26 (8H, m, aryl).
Lead Reference
Asymmetric synthesis of allylic secondary alcohols: a new general approach for the preparation of α-amino acids, Lorna J.Drummond, Andrew Sutherland, Tetrahedron, 2010, 66, 5349-5356 http://dx.doi.org/10.1016/j.tet.2010.05.066
Other References
Preparation, Properties, and Some Chemical Reactions of PhenaIeno [1,9-bc] furan, Gamini Weeratunga, Mona Austrup, and Russell Rodrigo, J. Chem. Soc. Perkin Trans. I, 1988, 3169-3173 http://dx.doi.org/10.1039/p19880003169
Keywords
acid chloride, carboxylic acids
Comments
in reaction wit thionyl chloride,
is anhydrous condition necessary??
By LeeSH on February 6, 2014