Hydrolysis of Ethyl 3-(1-pyrenyl)propanoate
SyntheticPage 513
DOI:
Submitted: October 6, 2011, published: October 11, 2011
Authors
Anish Mistry (a.mistry@warwick.ac.uk)
A contribution from
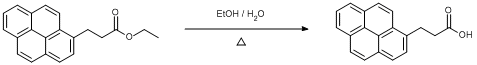
Chemicals
Ethyl 3-(1-pyrenyl)propanoate (1 equiv, prepared)
KOH (2 equiv, Fischer Scientific)
Ethanol
pH2 buffer solution (prepared)
KOH (2 equiv, Fischer Scientific)
Ethanol
pH2 buffer solution (prepared)
Procedure
Under a dinitrogen atmosphere, ethyl 3-(1-pyrenyl)propanoate (0.5 g, 1.6 mmol) was dissolved in warm ethanol (10 ml), then water (1 ml) and solid potassium hydroxide (0.18 g, 3.2 mmol) was added and the solution left stirring overnight. The reaction mixture was cooled and solvent removed under vacuo. Buffer solution (pH 2, 10 ml) was added and the mixture was extracted with ethyl acetate (10 ml x 3). The combined organics were dried over Na2SO4 and solvent was removed in vacuo to yield orange crystalline solid (0.45 g, 99%)
Author Comments
The reaction most probably takes less time (a couple of hours or so) however I just left it overnight to be sure.
Data
δH(400MHz, CDCl3) ppm: 2.96 (2H, t, J 8, CH2), 3.74 (2H, t, J 8, CH2), 7.95 (1H, d, J 8, aryl), 8.01 - 8.28 (7H, m, aryl), 8.29 (1H, d, J 9, aryl).
Lead Reference
Ta-Hsien Chuang, Shiow-Ju Lee, Cheng-Wei Yang and Pei-Lin Wu, Expedient synthesis and structure–activity relationships of phenanthroindolizidine and phenanthroquinolizidine alkaloids, Org. Biomol. Chem., 2006, 4, 860–867
Other References
(1-Pyrenylmethyl)amino alcohols, a new class of antitumor DNA intercalators. Discovery and initial amine side chain structure-activity studies, Kenneth W. Bair, Richard L. Tuttle, Vincent C. Knick, Micheal Cory and David D. McKee, Journal of Medicinal Chemistry, 1990, 33(9), 2385-93.
Keywords
esters, hydrolysis