Reduction of an imine to an amine
SyntheticPage 30
DOI:
Submitted: July 13, 2001, published: July 15, 2001
Authors
Lisa Titcomb (lisa.titcomb@gmail.com)
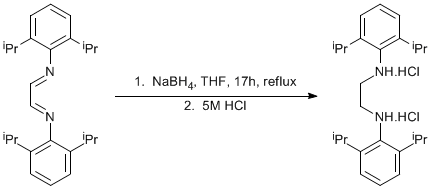
Chemicals
Glyoxal-bis-(2,6-diisopropylphenyl)imine (see Page 28)
Sodium Borohydride (Aldrich, stored in a glove box)
THF (heated at reflux over potassium for three days then distilled and stored in an ampoule over sieves)
Sodium Borohydride (Aldrich, stored in a glove box)
THF (heated at reflux over potassium for three days then distilled and stored in an ampoule over sieves)
Procedure
Glyoxal-bis-(2,6-diisopropylphenyl)imine (35.27g, 0.094mol) and NaBH4 (14.3g, 0.376mol) were placed into a two necked round bottom flask with a stirbar. The flask was fitted with a condenser and a hosing adapter with a teflon stopcock was attached to the top of the condenser. The apparatus was attached to a double manifold Schlenk line and cycled vac/argon three times and left open to argon. THF (300 mL) was added via canula. An oil bubbler was attached to a second tap of the double manifold and oppened to argon. The mixture was heated at reflux for 17h under an oil bubbler pressure of Ar and then cooled to ambient temperature. 5M HCl (300 mL) was added dropwise until fizzing had ceased and a white precipitate had formed. The solid was collected on a frit and washed with water (100 mL) and Et2O (200 mL). The solid was dried in a vacuum oven at 45 oC for 17h to yield 37.33 g of product (88% yield).
Author Comments
This procedure works well provided that the reaction mixture is heated at reflux for 17h rather than stirred for 16h and refluxed for 2h. Prolonged heating of the product under vacuum is necessary to ensure complete removal of water. The free diamine may be obtained by slurrying the dihydrochloride salt (1g) in water (50mL) and adding potassium carbonate (6g) then stirring for 15mins. Extraction with Et2O (3x50mL), drying with magnesium sulphate and removal of the solvent yields the free amine (0.820g, 98% yield).
Data
1H NMR (D6-DMSO): 1.16 (d, 24H, iPrCH3, 3J(HH) = 6.3 Hz), 3.36 (m, 4H, iPrCH, 3J(HH) = 6.3 Hz), 3.50 (s, 4H, CH2), 7.28 (m, 6H, aryl-CH).
Lead Reference
Arduengo, A. J.; Krafczyk, R.; Schmuter, R. Tetrahedron, 1999, 55, 14523.
Other References
For next stage in carbene prep see Page 31
Keywords
amine, imine, nucleophilic, reduction, sodium borohydride
Comments
The rigorous conditions are not necessary for good yields. To stirred imine in THF (distilled from Na/benzophenone), NaBH~4~ was added portionwise over 40 minutes. I stirred for 16 hours then refluxed for 6 hours, working up as above (70%). It is also worth noting that the yellow colour of the imine does not disappear until the mixture is worked up, which is worrying but unimportant.
By Andrew McCarroll on September 4, 2001