Ester reduction
SyntheticPage 99
DOI:
Submitted: August 17, 2001, published: August 17, 2001
Authors
Marco Soscia (m.g.soscia@sussex.ac.uk)
A contribution from
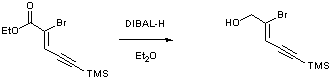
Chemicals
Dibal-H (aldrich)
Et2O (distilled over Na)
Et2O (distilled over Na)
Procedure
To a solution of ester (0.65 g, 2.36 mmol, 1 equiv.) in ether (71 mL) cooled to –78 oC was added DIBAL-H (0.92 mL, 5.17 mmol, 2.2 equiv.) dropwise. After 15 minutes the reaction flask was placed in an ice bath and stirring continued for a further 3 h. The reaction was quenched by the addition of Rochelles salt solution (40 mL) until effervescence ceased. Ether (50 mL) was added to the gum and stirring continued for a further hour. The organics were extracted into ether (4 x 50 mL) and washed with water (50 mL), dried (MgSO4), filtered and concentrated in vacuo yielding a yellow oil. Purification of this oil was achieved by chromatography on silica gel with petrol :ethyl acetate (6:1 v:v) as the eluent yielding the title compound as a yellow oil (0.445 g, 81 %).
Author Comments
This reaction was carried out several times and proved to be non-problematic.It was scaled up from 1 g - 12 g.
Data
1H: 6.35 (s, 1H), 4.35 (s, 2H), 2.37(brs, 1H), 0.26 (s, 9H)
Lead Reference
S.Caddick, V.M.Delisser, V.E.Doyle, S.Khan, Tetrahedron, 1999, 2737-2754