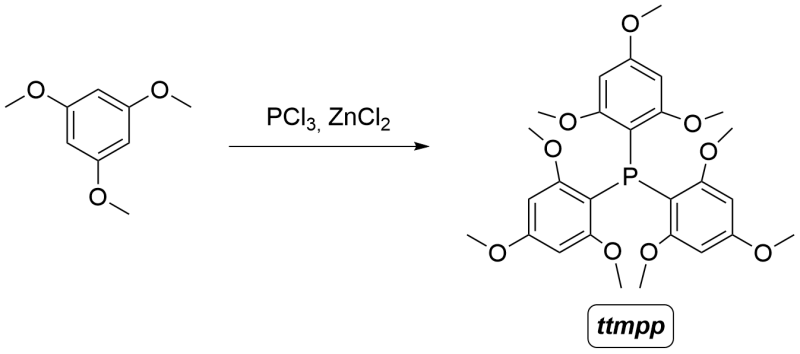
A simple and cheap synthesis of Tris(2,4,6-trimethoxyphenyl)phosphine (TTMPP)
SyntheticPage 965
Submitted: November 4, 2024, published: December 3, 2024
Authors
Emanuel Bruno Savini (emanuelbruno.savini@gmail.com)
Chemicals
Phosphorous trichloride - PCl3 - [7719-12-2] - ≥99%, Reagent grade (Sigma-Aldrich)
Zinc chloride - ZnCl2 - [7646-85-7] - ≥98%, (Sigma-Aldrich)
Toluene - C7H8 - [108-88-3] - ≥99,5%, ACS reagent (Sigma-Aldrich)
Cyclohexane - C6H12 - [110-82-7] - ≥99% (Sigma-Aldrich)
Ammonia solution - NH3 - [1336-21-6] - 15% in water - prepared from ACS reagent, 28.0-30.0% NH3 basis (Sigma-Aldrich)
Procedure
In a 10 mL round-bottom flask or Schlenk flask equipped with a magnetic stir bar, add 1,3,5-Trimethoxybenzene (673 mg, 4.00 mmol). Connect the flask to a nitrogen line to maintain an inert atmosphere.
In a large test tube, place an excess of zinc chloride and heat it for a few minutes with a heat gun or Bunsen burner until vapor evolution ceases to remove any water present in the salt. Allow the tube to cool under a gentle flow of nitrogen, then break the tube once it has reached room temperature and quickly weigh out anhydrous zinc chloride (182 mg, 1.34 mmol). Add this to the flask. Finally, add phosphorus trichloride (350 μL, 4.00 mmol) and bring the reaction to 90°C with a condenser attached.
After 8 hours at 90oC, stop the reaction by turning off the heating and by adding 5 mL of toluene, then stir quickly for a few minutes, and decant the solvent, leaving a viscous product (the TTMPP*ZnCl₂ complex) in the flask.
Repeat the toluene wash once again, then add approximately 5 mL of 15% ammonia solution.
Transfer the mixture, including all solids, into a small separatory funnel, add 5 mL of brine, and extract the aqueous phase thrice with 5mL of a 3:1 toluene-acetone mixture.
The combined organic washes are directly distilled on a rotary evaporator without drying (toluene-water azeotrope) yielding the final product as a pure white to off white solid.
The presence of remaining traces of trimethoxybenzene not removed effectively from the toluene washing can be detected by TLC (10-20% EtOAc/Petroleum ether. Phosphine remains at the baseline); this impurity is easily removed by washing the product thrice with 3mL of cyclohexane or pentane.
The yield is typically around 30-40%. Despite the low yield, the reaction is highly selective, with minimal formation of side products, allowing for almost complete recovery of unconverted trimethoxybenzene from the initial toluene washings.
Author Comments
- PCl3 and Trimethoxybenzene were both distilled before performing the reaction, this reduces quite a bit colored impurities in the final product, but does not affect heavily the yield of the transformation.
- Clumping is a significant limiting factor for the success of the reaction. Ensure that the stir bar does not stop frequently during the process, and carefully select the appropriate size of the stir bar based on the flask being used. On a small scale, using a Schlenk tube can help minimize clumping issues due to its narrow shape.
- Unconverted trimethoxybenzene can be recovered by washing in a separatory funnel the combined toluene extracts (from the crude TTMPP*ZnCl₂ complex) with 5mL of 15% ammonia, 5mL of water, 5mL of brine and drying the organics with magnesium sulphate. The toluene estract is than charged on a wide enough pad of silica and washed with a copious amount of ethyl ether. The solution is evaporated at reduced pressure to yield the pure starting material.
- NMR spectra were collected on Varian Inova 600MHz NMR and Varian Oxford 400MHz NMR.
- The author sincerely thanks Professor M. Fochi and Professor L. Bernardi.
Data
31P NMR (243 MHz, CD3CN) δ -67.98.
Lead Reference
Supplementary Information
Keywords
electrophilic, organophosphorous, phosphines, substitution