Dechlorination followed by hydrolysis of Clobazam
SyntheticPage 960
Submitted: May 8, 2023, published: June 7, 2023
Authors
Gangadhar Meti (gymeti@rediff.com)
Gopala Krishna Rao (gkfadnis@gmail.com)
Heena Beig (heenabeig@gmail.com)
Ramesha Ramakrishna (ramesha63@hotmail.com)
A contribution from
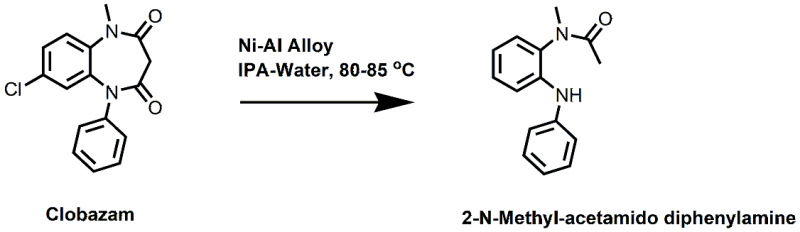
Chemicals
1. 7 chloro-1-methyl-5-phenyl-1,4-benzodiazepine-2,3 dione: prepared as per patent No: US 3984398
2. Nickel/Aluminium alloy: SD Fine Chem
3. Sodium carbonate: Commercial
4. Isopropyl alcohol (IPA): SD Fine Chem
5. DM Water: Commercial
6. Methylene Di chloride(MDC): SD Fine Chem
Procedure
Author Comments
2. Spent Ni after the reaction is pyrophoric. Should be immediately kept under water
Data
1H NMR:(CDCl3δ ppm):1.92(3H,Doublet), 3.22(3H,Quartet),6.55(1H,Singlet), 6.82-7.39(8H, Ar-H)
13C NMR:(CDCl3δ ppm):134.8(C-Cl),140.6,141.8(C-NH),171.8(C=O)
Lead Reference
Other References
Supplementary Information
Keywords
amides, aromatics/arenes, carbamates, Clobazam, Dechlorination, diphenylamine, hydrogenation, Ni-Al alloy, reduction