Esterification of Monomethyl Fumarate
SyntheticPage 959
Submitted: August 3, 2022, published: October 5, 2022
Authors
A contribution from
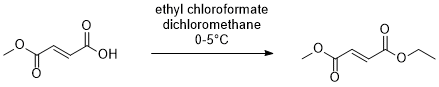
Chemicals
1. Monomethylfumaric acid: Prepared as per patent No: WO2021/053476A1
2. Dichloromethane: Commercial
3. Ethylchloroformate: Aldrich
4. Sodium sulfate: SD Fine Chem
5. Triethylamine: SD Fine Chem
Procedure
In a 500 mL RB flask fitted with mechanical stirrer, nitrogen inlet and addition flask, dichloromethane (150 mL) was charged followed by mono methylfumaric acid (25 g, 0.192 mol) at room temperature followed by triethylamine (29.2 g, 0.288 mole). Reaction mixture was cooled to about 2-3 degree centigrade under nitrogen atmosphere. At this temperature, ethylchloroformate (23 g, 0.211 mole) in 50 mL dichloromethane was slowly added keeping the temperature below 5 oC. After 1 h of stirring, cooling was removed and allowed to come to RT in 2h. TLC (7:3, ethyl acetate: cyclohexane, Rf:~0.8), showed complete conversion of monomethyl fumarate to ethyl methyl fumarate . Slowly added the reaction mixture to water 200 mL and separate dichloromethane layer. Water wash 2 times (2x 100 mL) and evaporated the dichloromethane layer to get ethyl methyl fumarate (30 g, 98.7 %) which is pure by NMR.
Author Comments
1. Dry condition is required for succesful reaction. 2. Product is almost pure by NMR (attached). 3. 1H NMR and 13C NMR attached
Data
1H NMR (CDCl3, delta): 6.86(2H, Singlet), 4.28-4.23(2H, Quartet), 3.80(3H, Singlet), 1.33-1.30 (3H, Triplet)
13C NMR (CDCl3, delta): 165.12, 164.59, 133.63, 132.28, 61.06, 51.97, 13.79
Lead Reference
Patent No: WO2021/053476A1
Keywords
addition, carboxylic acids, electrophilic, Esterification, esters, Ethyl, Ethyl chloroformate, Fumarate, insertion, Methyl