Dehydration and Cyclisation of N-Phenylanthranilic acid
SyntheticPage 955
Submitted: November 18, 2021, published: January 5, 2022
Authors
Robert Smith (rbsmith@uclan.ac.uk)
Sarah L. Brennan
A contribution from
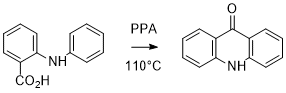
Chemicals
Polyphosphoric acid (Sigma Aldrich)
Procedure
Author Comments
* A 600 ml beaker was used due to the very dense and gloopy nature of polyphosphoric acid. A minimum amount of acid was used to cover surface of fine solid and this is always in excess due to the nature of the reaction. It should be noted that if the N-phenylanthranilic acid is wet from the previous synthesis, steam will be generated as the polyphosphoric acid is heated. The large sided beaker helps to control this.
** The termperature was controlled using a sensor on the hotplate. Constant stirring was maintained using a stirrer bar and controlled via the hot plate. The reaction did not require dry/inert conditions (as water is generated as a by-product) and took place in open air.
*** Other methods of purification could involve piyring the reaction slowly into a stirring solution of hot water (80°C) rather then over ice. Although this works, its can generate a vigourous reaction and serious consideration needs to be given to going down this route based on safety aspects.
Data
Lead Reference
Litchfield, V.J., Smith, R.B., Franklin, A.M., Davis, J., Synthesis of acridine-quinone systems - A potential electrochemical fluorescent switch (2008) Synthetic Communications, 38 (20), pp. 3447-3455.
Supplementary Information
Keywords
aromatics/arenes, carboxylic acids, heterocyclic compounds