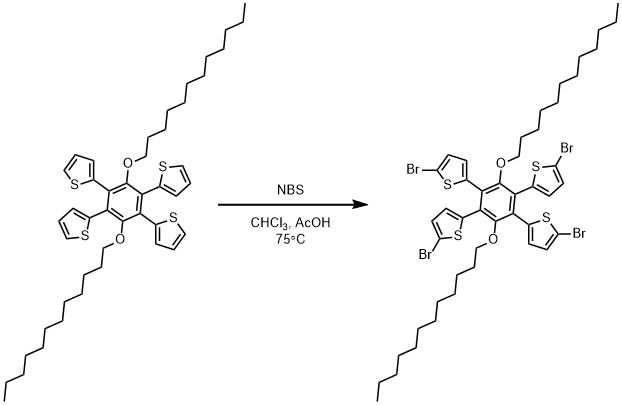
Bromination of 2,2',2'',2'''-(3,6-bis(dodecyloxy)benzene-1,2,4,5-tetrayl)tetrathiophene
SyntheticPage 953
Submitted: July 20, 2021, published: July 28, 2021
Authors
Kathryn Allen (kathryn.allen@millersville.edu)
Landon Kurtz (lmkurtz@millersville.edu)
A contribution from
Chemicals
2,2',2'',2'''-(3,6-bis(dodecyloxy)benzene-1,2,4,5-tetrayl)tetrathiophene
Acetic acid, glacial
Chloroform, Supelco
N-Bromosuccinimide 99%, AldrichProcedure
A three-necked round-bottomed flask was fitted with a stir bar and a condenser. Chloroform (4mL) and acetic acid (4mL), 2,2',2'',2'''-(3,6-bis(dodecyloxy)benzene-1,2,4,5-tetrayl)tetrathiophene (0.250 g, 0.322 mmol, 1 eq.) and N-bromosuccinimide (0.252 g, 1.417 mmol, 4.4 eq.) were added to the three-necked flask and the reaction was heated in an oil bath at 75° Celsius. The reaction was monitored with 1H NMR to check for reaction completion. Upon completion, the reaction was cooled and diluted with chloroform, washed with saturated sodium thiosulfate (75mL, 3x) and saturated sodium chloride (75mL, 3x). The organic layer was dried over magnesium sulfate and filtered, concentrated in vacuo, and taken up in hot methanol. Upon cooling, the product was separated by filtration. The product was confirmed by thin layer chromatography (hexanes, Rf = 0.17) and was collected as a brown powder (0.263 g, 0.241 mmol, 75% yield).
Author Comments
Acetic acid greatly accelerates this reaction. Brominating in chloroform yielded little to no product. The reaction was generally done in five hours, but was capable of over-bromination!
Please note: NBS must be recrystallized from water before being used in order to receive the best results.
Data
1H NMR (400 MHz, CDCl3): δ 6.887 (d, 4H, J = 4.0 Hz), 6.618 (d, 4H, J = 3.6 Hz), 3.231 (t, 4H, J = 6.4 Hz), 1.220 (m, 32H), 0.992 (m, 8H), 0.880 (t, 6H, J = 6.8 Hz)
13C NMR (400 MHz, CDCl3): δ 152.276, 137.605, 129.826, 129.591, 129.272, 113.448, 73.642, 31.923, 29.836, 29.684, 29.653, 29.608, 29.524, 29.357, 29.198, 25.631, 22.686, 14.118.ESI positive mode on AXION 2 Time of Flight Mass Spectrometer
Calculated [M+H]: 1091.0090
Found [M+H]: 1091.0116
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes