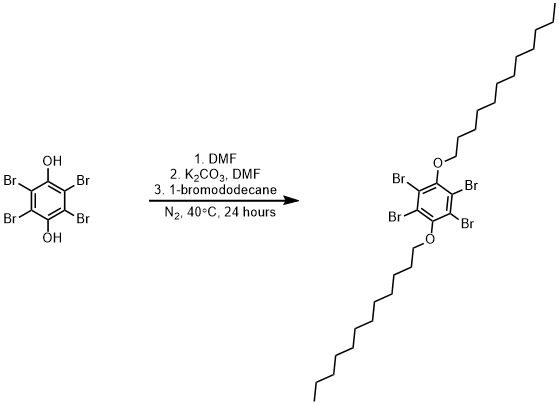
Alkylation of 2,3,5,6-tetrabromobenzene-1,4-diol with bromododecane
SyntheticPage 951
Submitted: July 20, 2021, published: July 23, 2021
Authors
Kathryn Allen (kathryn.allen@millersville.edu)
Landon Kurtz (lmkurtz@millersville.edu)
A contribution from
Chemicals
1,2,4,5-tetrabromobenzene-1,4-diol, 97%
1-Bromododecane, 97% Aldrich
Dimethylformamide, anhydrous Macron Fine Chemicals
Potassium carbonate, VWRProcedure
Two dry three-necked round-bottom flasks were fitted with magnetic stir bars and septa and purged with nitrogen gas for fifteen minutes. 1,2,4,5-tetrabromobenzene-1,4-diol (0.500 g, 1.174 mmol, 1 eq.) was added to one of the flasks and potassium carbonate (0.389 g, 2.818 mmol, 2.4 eq.) was added to the other flask. Dimethylformamide (8mL x 2) was added to each flask. Each slurry was degassed with nitrogen for 2 hours to remove oxygen. The 2,3,5,6-tetrabromobenzene-1,4-diol solution was then syringed into the potassium carbonate slurry. 1-Bromododecane (0.675 mL, 2.818 mmol, 2.4 eq.) was added dropwise and the reaction was heated for 26 hours at 40° Celsius. The product was verified by thin layer chromatography (hexanes, Rf = 0.47). The reaction was then cooled to room temperature, poured into 50 mL of ice cold water, and filtered. The crude solid was recrystallized from hot methanol to yield the product as a fluffy white solid (0.676 g, 0.887 mmol, 75% yield).
Author Comments
This synthesis is very sensitive to air, and degassing for extended periods of time drastically improved yield.
The peak on the 13C NMR at 29.642 ppm did not show due to resolution issues, but should be noted it is present.
The peak on the 13C NMR at 29.642 ppm did not show due to resolution issues, but should be noted it is present.
Data
1H NMR (400 MHz, CDCl3): δ 3.964 (t, 4H, J = 6.8 Hz), 1.869 (m, 4H), 1.504 (m, 4H), 1.352 (m, 32H), 0.883 (t, 6H, J = 7 Hz)
13C NMR (400 MHz, CDCl3): δ 151.927, 121.387, 73.573, 31.923, 29.889, 29.661, 29.642, 29.623, 29.578, 29.441, 29.365, 25.821, 22.701, 14.141ESI positive mode on AXION 2 Time of Flight Mass Spectrometer
Calculated [M]: 762.0501
Found [M]: 762.0489
Keywords
alcohols, ethers, nucleophilic, substitution