Transetherification of 3-methoxythiophene
SyntheticPage 950
Submitted: February 26, 2021, published: February 27, 2021
Authors
Colette Sullivan (colettes@usca.edu)
Nicholas Marshall (nicholasm@usca.edu)
A contribution from
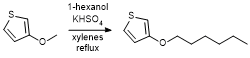
Chemicals
3-methoxythiophene, AmBeed
1-hexanol, 99.9%, Alfa Aesar
Potassium Bisulfate (KHSO4), J.T. BakerProcedure
3-methoxythiophene (2.74 g, 2.4 mL, 24 mmol), 1-hexanol (2.95 g, 2.40 mL, 28.8 mmol), and potassium bisulfate (0.392 g, 2.88 mmol) were suspended in 30 mL xylenes in a 100 mL round bottom flask. The reaction flask was fitted with a condenser and heated to reflux under nitrogen over 48h. The flask was fitted with a Claisen-style still head incorporating a Vigreux condenser attached to a water-cooled condenser. Xylenes and hexanol were removed by distillation under nitrogen until the vapor temperature reached 185°C under atmospheric pressure. Then, house vacuum (1-5 Torr) was applied and product was collected by vacuum distillation of the remaining liquid in the reaction flask, 2.3 g total (52%).
Author Comments
After 30 minutes of refluxing, trace amounts of product were seen via TLC.
In one case while running this reaction, no significant conversion was seen after refluxing for the indicated time. We drove the reaction to completion by a second addition of 1-hexanol and KHSO4 (in the amounts in the original procedure). TLC indicated completion after an additional 12 hours of reaction time.Data
1H NMR (60 MHz): 0.999 (t, 3H), 1.47 (m, 8H), 3.98 (t, 2H), 6.28 (m, 1H), 6.86 (m, 1H), 7.20 (m, 1H).
Lead Reference
Xu, Noh, and Thompson, Macromolecules, 2014, 47, 5029-5039, https://pubs.acs.org/doi/10.1021/ma5012107
Supplementary Information
Keywords
alcohols, alkoxythiophene, aromatics/arenes, ethers, thiophene