Conversion of a vinyl bromide to a vinyl iodide by conjugate addition-elimination
SyntheticPage 95
DOI:
Submitted: August 17, 2001, published: August 17, 2001
Authors
Marco Soscia (m.g.soscia@sussex.ac.uk)
A contribution from
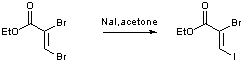
Chemicals
NaI (aldrich)
Acetone (analytical)
Acetone (analytical)
Procedure
Ethyl-2,3 dibromopropenoate (21.2 g, 82 mmol, 1 equiv.) was added to a stirred solution of sodium iodide (24.6 g, 0.164 mol, 2 equiv.) in acetone (80 mL). The resulting orange suspension was heated at reflux for 3 days. The resulting brown liquid was filtered to remove inorganics and concentrated in vacuo. The resulting brown oil was purified by chromatography on silica gel with petrol:DCM (4:1 v:v) as the eluent yielding the title compound as a red oil (23.5 g, 94 %)
Author Comments
This procedure has been carried out by numerous people in our lab in scales varying from 1g-50g.
This reaction was seen to be non-problematic.
Data
1H: 8.69(s, 1H), 4.22(q, 2H, J=7.12Hz), 1.27 (t, 3H, J=7.12Hz)
Lead Reference
Caddick, S.; Delisser, V. M.; Doyle, V. E.; Khan, S.; Avent, A. G.; Vile, S.; Tetrahedron, 55, 1999, 2737 - 2754.