Suzuki-Miyaura coupling reaction of potassium aryl trifluoroborate using palladium(II) acetate
SyntheticPage 948
Submitted: February 15, 2021, published: March 1, 2021
Authors
Takuya Miura (Takuya.Miura@TCIchemicals.com)
Yuko Konishi (Yuko.Konishi@tcichemicals.com)
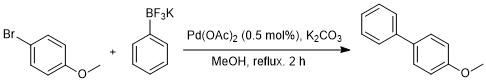
Chemicals
potassium phenyltrifluoroborate (TCI-P1582, used as received)
4-bromoanisole (TCI-B0547, used as received)
palladium(II) acetate (TCI-P2161, used as received)
potassium carbonate
methanol
water
dichloromethane
hydrochloric acid (2mol/L)
brine
anhydrous sodium sulfate
silica-gel
n-hexane
ethyl acetate
Procedure
Potassium phenyltrifluoroborate (500 mg, 2.72 mmol), potassium carbonate (1.13 g, 8.15 mmol), and palladium(II) acetate (3 mg, 0.014 mmol) were added to solution of 4-bromoanisole (509 mg,2.72 mmol) in methanol (10 mL) at rt under N2 atmosphere. After reflux for 2 h, the reaction mixture was cooled to rt, quenched by addition of water (20 mL), and extracted with dichloromethane (30 mL×2). Obtained organic phase was washed with 2N HCl aq. (30 mL) and brine (30 mL), dried over anhydrous sodium sulfate and filtered. After solvent was removed under reduced pressure, crude was purified by column chromatography (silica-gel, n-hexane:EtOAc = 20:1) to give 4-methoxybiphenyl (380 mg,76%) as a white solid.
Author Comments
All chemical manipulations were performed in a fume hood.
Methanol was used as reaction solvent without degassing operation.
Consumption of stating material and formation of product were monitored by TLC(n-hexane:AcOEt = 20:1). Rf: 0.625(4-bromoanisole), 0.50(4-methoxybiphenyl).
This procedure is typical for Suzuki-Miyaura coupling using organotrifluoroboates.
Methanol was used as reaction solvent without degassing operation.
Consumption of stating material and formation of product were monitored by TLC(n-hexane:AcOEt = 20:1). Rf: 0.625(4-bromoanisole), 0.50(4-methoxybiphenyl).
This procedure is typical for Suzuki-Miyaura coupling using organotrifluoroboates.
Data
1H NMR (400 MHz, CDCl3); δ 7.51–7.56 (m, 4H), 7.39–7.44 (m, 2H), 7.28–7.32 (m, 1H), 6.96–6.99 (m, 2H), 3.85 (s, 3H).
Lead Reference
G. A. Molander and B. Biolatto, J. Org. Chem. 2003, 68, 4302. (DOI: https://doi.org/10.1021/jo0608366)
Supplementary Information
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes, cross coupling, transition metal catalysed