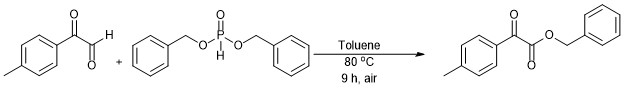
Synthesis of Benzyl 2-oxo-2-(p-tolyl)acetate through 2-Oxo Promoted Hydrophosphonylation followed by Aerobic Intramolecular Nucleophilic Displacement Reaction
SyntheticPage 946
Submitted: January 20, 2021, published: February 22, 2021
Authors
Satyanarayana Battula (satyamssd@gmail.com)
Shivani, N. Tandel (shivanitandel1929@gmail.com )
A contribution from
Chemicals
(1) 4-Methyl phenylglyoxal [synthesized from 4-methyl acetophenone1 (Sigma Aldrich), 97%, 142433]
(2) Dibenzyl phosphite (Sigma Aldrich), 100%, D36607
(3) Anhydrous sodium sulfate (Rankem), 99%, S0410
(3) Toluene (Rankem), directly used without drying
(4) n-Hexane (SD Fine Chemicals Ltd), directly used without drying
(5) Ethyl acetate (SD Fine Chemicals Ltd), directly used without drying
Procedure
Reaction vessel was charged with 4-methyl phenyl glyoxal (77 mg, 0.46 mmol) and dibenzyl phosphite (111 µL, 0.506 mmol) in 2 ml of Toluene and fitted with a reflux condenser and the reaction mass was heated for 7-9 h at 80 oC. The progress of the reaction was monitored by TLC, after its completion, the reaction mass was cooled to room temperature and excess solvent was removed under reduced pressure. The reaction mass was extracted with ethyl acetate from its aqueous workup. The EtOAc extract was dried over anhydrous Na2SO4. The it was purified by column chromatography on silica gel (100-200 #) using hexane and ethyl acetate (97:3) as eluent to afford the corresponding product with 75% yield at 90% purity.
Author Comments
- Due to 2-oxo group, the assistance of H-bonding, the dialkyl H-phosphonate form shifts towards more nucleophilic phosphite form.
- The phosphite group later attacks 4-methyl phenyl glyoxal and generates the desired product and on heating in toluene loses one proton that generates a resonance stabilized caranion.
- This carbanions later attacks on molecular oxygen, and that rearranges to dioxaphosphetane intermediate and releases alkoxy nucleophile.
- Under in situ acidic environment the dioxaphosphetane undergoes nucleophilic displacement reaction through elimination of H2O and PO3R molecules.
Data
1H NMR: (400 MHz, CDCl3) δ 7.78 (d, J = 7.9 Hz, 2H), 7.41-7.26 (m, 5H), 7.20 (d, J = 7.9 Hz, 2H), 5.33 (s, 2H), 2.35 (s, 3H)
13C NMR:(126 MHz, CDCl3) δ 185.77, 163.88, 146.39, 134.61, 130.21, 129.96, 129.68, 128.93, 128.82, 128.77, 128.64, 67.71, 21.98
IR (CHCl3, cm-1) ν 2922, 2852, 1737, 1681, 1303, 1169, 999, 696.
Lead Reference
Battula, S.; Battini, N.; Singh, D.; Ahmed, Q. N., 2-Oxo Promoted Hydrophosphonylation & Aerobic Intramolecular Nucleophilic Displacement Reaction. Org. Biomol. Chem. 2015, 13, 8637-8641.
https://doi.org/10.1039/C5OB01310KOther References
- Riley, H. A.; Gray, A. R. Org. Synth. Coll. 1935, 15, 67.
- Huang, Z.; Wang, K.-K. A.; Lee, J.; Donk, W. A. v. d., Chem. Sci. 2015, 6,1282-1287. https://doi.org/10.1039/C4SC03095H
- Zhang, C.; Feng, P.; Jiao, N., J. Am. Chem. Soc. 2013, 135, 15257-15262. https://doi.org/10.1021/ja4085463
- Eftekhari-Sis, B.; Zirak, M., Chem. Rev. 2015, 115, 151-264. https://doi.org/10.1021/cr5004216
Supplementary Information
Keywords
2-Oxoester, esters, nucleophilic, phosphonylation, substitution, thermal