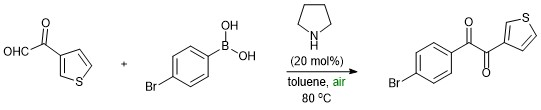
Synthesis of 1-(4-bromophenyl)-2-(thiophen-3-yl)ethane-1,2-dione through 2-oxoiminium mediated oxidative cross coupling reaction
SyntheticPage 945
Submitted: January 15, 2021, published: February 22, 2021
Authors
Bhavyesh Desai (bhavyeshdesai42@gmail.com)
Satyanarayana Battula (satyamssd@gmail.com)
A contribution from
Chemicals
1. (4-bromophenyl)boronic acid (Sigma-Aldrich), ( 95.0%), B75956
2. 2-Oxo-2-(thiophene-3-yl)acetaldehyde (Synthesized from 3-acetylthiophene,1 Sigma-Aldrich, 98%, 196320)
3. Pyrrolidine (Sigma-Aldrich), (99%), P73803
4. Toluene (Rankem), directly used without drying
5. Ethyl acetate (SD Fine Chemicals Ltd), directly used without drying
6. Hexane (SD Fine Chemicals Ltd), directly used without dryingProcedure
Author Comments
- Initially 2-Oxo-2-(thiophene-3-yl)acetaldehyde reacts with pyrrolidine to form 2-oxoiminium ion which can differentiate the regular iminium in the reactivity.
- Carbonyl group in 2-oxoimminium ion increases its reactivity, and thus undergoes nucleophilic [1,2] addition of boronic acid followed by in situ abstraction of proton (boric acid), deamination and aerobic oxidation to form the final dione product.
- α-Ketoamide is formed in the absence of boronic acid nucleophile.
- Generally, this sort of coupling reaction requires harsh reaction conditions.
Data
1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 2.8, 1.2 Hz, 1H), 7.93 – 7.83 (m, 2H), 7.70 – 7.62 (m, 3H), 7.41 (dd, J = 5.1, 2.9 Hz, 1H);
13C NMR (126 MHz, CDCl3) δ 191.91, 186.39, 137.74, 137.34, 137.25, 132.31, 131.50, 130.42, 127.33, 127.15
GC-MS (EI) m\z (relative intensity): 294.0 (M+, 2.6), 183.1 (25), 155.2 (11), 111.2 (100), 83.0 (8.4).
Lead Reference
N. Mupparapu, N. Battini, S. Battula, S. Khan, R. A. Vishwakarma, Q. N. Ahmed, Aminocatalytic Cross-Coupling Approach via Iminium Ions to different C-C Bonds. Chem. Eur. J. 2015, 21, 2954-2960
DOI: https://doi.org/10.1002/chem.201405477.Other References
- Riley, H. A.; Gray, A. R. Org. Synth. Coll. 1935, 15, 67.
- M. Wasa, R. Y. Liu, S. P. Roche, E. N. Jacobsen, J. Am. Chem. Soc. 2014, 136, 12872-2875.
https://doi.org/10.1021/ja5075163
- G. D. Vo, J. F. Hartwig, J. Am. Chem. Soc. 2009, 131, 11049-11061.
https://doi.org/10.1021/ja903049z
- L. Huang, K. Cheng, B. Yao, Y. Xie, Y. Zhang, J. Org. Chem. 2011, 76, 5732 –5737
Supplementary Information
Keywords
2-oxoiminium ion, aldehydes, amines, dione, heterocyclic compounds, nucleophilic, oxidative addition, thermal