One-Pot Synthesis of MK-9
SyntheticPage 944
Submitted: January 9, 2021, published: February 10, 2021
Authors
Nanaji Yerramsetti (naniorg@gmail.com)
Nitin Patel (nitin@aether.co.in)
Satyanarayana Battula (satyamssd@gmail.com)
A contribution from
Chemicals
1. Solanesol (Sigma-Aldrich) >90%, S8754
2. Diels-Alder adduct of menadione
3. Ghosez reagent (Sigma-Aldrich) 96%, 498270
4. tBuOK (Sigma-Aldrich) 98%, 156671
5. Toluene (Rankem), directly used without drying
6. 8-Crown-6 (Sigma-Aldrich) 99%, 186651
7. AcOH (Sigma-Aldrich) >99%, A6283
8. Bu3MeNBr (Sigma-Aldrich) >98%, 70444
9. Ethyl acetate (SD Fine Chemicals Ltd), directly used without drying
10. Hexane (SD Fine Chemicals Ltd), directly used without drying
Procedure
Author Comments
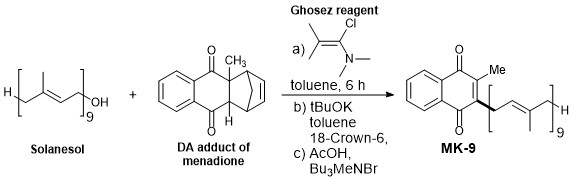
- Ghosez reagent is used here to generate efficiently solanesyl chloride from solanesol.
- Using less efficient leaving group/ more efficient nucleophile (Cl) in the solanesyl chloride makes it more prominent reaction and causes low possibilities of side reactions.
- Solanesyl chloride coupled with Diels-Alder adduct of menadione followed by the elimination of cyclopentadiene to produce MK-9.
- The menaquinone MK-9 in this procedure was obtained as an exclusive diastereomer with significant yield (77%, 99.9% purity by chiral HPLC).
Data
1H NMR (400 MHz, CDCl3): δ (ppm) 8.89−8.39 (m, 2H), 7.64−7.98 (m, 2H), 5.22−4.98 (m, 9H), 3.36 (d, J = 7.2 Hz, 2H), 2.80 (s, 3H), 2.18−1.88 (m, 37H), 1.78 (s, 3H), 1.66 (s, 3H), 1.60-1.48 (m, 19H).
m.p.: 50.8 - 61.6 °C.
Lead Reference
N. Yerramsetti, L. Dampanaboina, V. Mendu, and S. Battula, Synergistic Factors Ensue High Expediency in the Synthesis of Menaquinone [K2] Analogue MK-6: Application to Access an Efficient One-Pot Protocol to MK-9. Tetrahedron, 2020, 76, 131696.
Other References
- A. Rüttimann, Recent Advances in the Synthesis of K-Vitamins. Chimia 1986, 40, 290–306.
- A. Baj, P. Walejko, A. Kutner, L. Kaczmarek, J. W. Morzycki, S. Witkowski, Org. Process Res. Dev, 2016, 20, 1026-1033.(https://doi.org/10.1021/acs.oprd.6b00037)
- F. Munyemana, I. George, A. Devos, A. Colens, E. Badarau, A. -M. Frisque-Hesbain, A. Loudet, E. Differding, J.-M. Damien, J. Remion, J. V. Uytbergen, L. Ghosez, Tetrahedron 2016, 72, 420-430. (https://doi.org/10.1016/j.tet.2015.11.060)
Supplementary Information
Keywords
alcohols, alkyl/alkenyl/aryl halides, nucleophilic, substitution, terpenoid, thermal, Vitamin K2